Everyone loves the smell of fresh bread and wine, but the secret behind them is a fungus—yeast. Yeast is a critical ingredient for making bread and wine and has been used for thousands of years. However, not all yeasts are suitable for human consumption. Which of these should we use? We look at the differences between baker’s yeast, wine yeast and wild yeast.
All yeast strains perform fermentation. However, baker’s yeast produces more carbon dioxide, while wine yeast can tolerate higher ethanol concentrations. Wild yeasts are unpredictable and can create atypical chemicals, some of which lend new flavors and aromas to bread and wine.
What is Yeast?
Fermentation Experts
Yeast is a one-celled organism belonging to the fungi kingdom. You can find them almost everywhere, in fruits, grains, or any sugar-rich environment. It is also humankind’s oldest ingredient in making bread and wine.
Yeast cells perform fermentation, which involves breaking down sugars into alcohols and carbon dioxide gas. For example, certain yeasts break down glucose (a sugar) into ethanol (an alcohol):
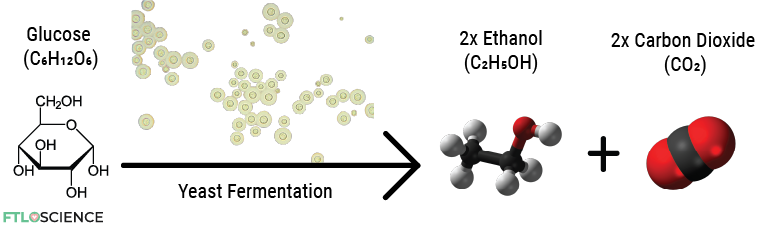
Over 2000 species of yeast are known, with Saccharomyces cerevisiae being the most commonly used in baking and brewing. Different strains of S. cerevisiae find use in various commercial applications.
Saccharomyces cerevisiae
Yeasts can be commercially bought or found in the wild. Most bakers and winemakers go the commercial route as they can choose from hundreds of genetically engineered S. cerevisiae strains to suit their needs.
A common strain of commercial baker’s yeast is S. cerevisiae Y234 due to its high gas production. Brewer’s (wine) yeast, like S. cerevisiae 4021, has a higher tolerance to ethanol, helping it survive in fermentation containers like wine casks.
There are ‘wild’ species of yeast that also perform fermentation, but they are difficult to control and can produce unpleasant tasting chemicals.
Is Wild Yeast Useful?
Commercial and wild yeast both ferment sugars to form alcohol and carbon dioxide. However, wild yeasts often differ in biological makeup, alcohol tolerance and chemical products in ways that we don’t yet fully understand.
Hence, large-scale manufacturers usually prefer specific strains for making bread and wine. However, smaller bakeries and breweries may experiment with wild yeast strains to impart different flavors and aromas to their food and beverages.
| Species (Strain) | Type | Primary Use | Alcohol Tolerance |
|---|---|---|---|
| S. cerevisiae (Y234) | Baker’s Yeast | Produces gas for dough to rise | 8% |
| S. cerevisiae (4021) | Wine Yeast | Increases ethanol content | 17% |
| Zygosaccharomyces bailii | Wild Yeast | Making kombucha | 15% |
Differences Between Yeast Strains
Chemistry and Biology
Commercial yeast often has more specialized proteins than wild yeast strains. Like humans, yeast cells need proteins to perform life-sustaining functions. The instructions for making these proteins come from their genes.
We can control the genetic makeup of commercial yeast strains, introducing genes that code for helpful proteins while removing genes responsible for non-essential or harmful proteins.
For example, baker’s yeast (S. cerevisiae Y234) has five copies of a gene that codes for a particular enzyme. This enzyme breaks down complex sugars efficiently into carbon dioxide gas.
Wild yeast strains usually contain fewer of these complex sugar enzymes. As a result, baker’s yeast ferments faster, allowing bread to rise in a short period.
Alcohol Tolerance
Wine yeast strains can tolerate higher levels of alcohol than wild yeast. Commercial wine yeast can withstand up to 17% alcohol without dying.
Wild yeasts have a range of alcohol tolerance values. Most species of wild yeast stop fermentation processes when alcohol concentration is at 6%, which limits the alcohol content of the brew.
Other wild yeasts like Zygosaccharomyces bailii (used in making kombucha) can survive in environments of up to 15% ethanol.
Chemical Aromas
Manufacturers use commercial yeasts that produce pleasant-smelling chemicals. These aromas include the characteristic scent of freshly-baked bread or the fruity and nutty aromas in wine.
It is more difficult to control the aromas that wild yeasts produce, which can include chemical scents of body odor or onions.
Baker’s Vs. Wine Vs. Wild Yeast: Which to Use?
Baker’s Yeast
In baking, we use yeast to help the dough rise, as they produce carbon dioxide gas through fermentation. Carbon dioxide accumulates in the dough and creates air bubbles, acting as a leavening that expands the dough. (You can learn all the chemical reactions of baking here)
Baker’s yeast should, therefore:
- Consume sugar in the batter to produce large quantities of carbon dioxide
- Produce as little ethanol (and other by-products) as possible
- Perform fermentation quickly, decreasing the time that the dough takes to rise
- Be easily killed by heat from the oven

One such strain is S. cerevisiae Y234, which ticks all the boxes. Bread baked with Y234 yeast is usually soft and has an airy feel.
Wild yeast can be introduced into the baking mix by adding fruits or herbs soaked in water, which gives the pastry certain (sometimes unpredictable) aromas. Unique varieties of bread, like sourdough, also incorporate bacteria that work with the yeast to produce lactic acid.
Wine Yeasts
Fermentation with wine yeast focuses on ethanol production to reach the alcohol content in an alcoholic beverage. Winemakers use specific wine yeast strains that are resistant to ethanol.
For example, if we use baker’s yeast to make wine, fermentation will stop when alcohol levels reach 6%, resulting in sweet, low-alcohol content wine (and dead yeast cells).

Depending on the specific alcohol content, different wine yeasts are used. For example, S. cerevisiae strain 4021 can tolerate up to 17% alcohol and ferments sugars efficiently while producing little foam, making it ideal for white wines.
Engineered wine yeasts are helpful for biofuel production, where we need to efficiently ferment inedible plant material (like rice husk) into ethanol—a sustainable supplement to gasoline.
Wild Yeasts
Yeast is a natural part of our environment, but it is considered a contaminant when it enters industrial fermentation processes. Many wild yeast species exist, each capable of fermenting a range of chemical compounds.
While they perform the usual fermentation of glucose into ethanol and carbon dioxide, wild yeasts can also produce different fermentation chemicals, some of which are unsafe.
For example, methanol in alcoholic drinks results from wild yeast entering non-sterile fermentation tanks and breaking down pectin, a sugar found in fruits (wine yeast cannot do this).
Many species of wild yeast can also decompose amino acids, producing fusel oils (alcohols heavier than ethanol) that cause more severe hangover symptoms.
In bread and wine making, wild yeasts can produce unpleasant odors. Chemicals from wild yeast fermentation are responsible for several ‘wine faults’, including the sour taste of acetic acid and acetaldehyde.
Adventurous winemakers sometimes mix wild yeast species like Kloeckera apiculata with wine yeast to create different flavors and aromas. However, these fermentation processes require constant monitoring as wild yeasts often grow more aggressively than baker’s and wine yeasts, out-competing them for nutrients.
The Wonderful World of Yeasts
If you think about it, yeasts are a little like dogs. Like we breed dogs for their shepherding or guarding abilities, we have perfected yeast strains that perform best in tasks like breadmaking and winemaking over thousands of years.
Today, we have so many different strains of yeast available to choose from, especially for winemaking and beverages. There are specific yeasts for reds, whites, ciders, ales and for producing nutty, fruity, acidic, sour flavors.
We hope you’ve learned something about different types of yeast. The next time you come across sourdough or kombucha, you can impress your friends with your knowledge of the differences between baker’s yeast, alcohol yeast and the wonderfully unpredictable wild yeasts!
About the Author

Marjory was a junior science writer at FTLOScience from October to November 2022.




