When formulating liquid drugs, we sometimes need them to be isotonic (have the same salt concentration as our body fluids) to prevent patient discomfort. This guide shows you how to calculate and adjust the tonicity of solutions using two different methods: sodium chloride equivalence (SCE) and freezing point depression (FD).
Isotonic solutions are important in liquid pharmaceutical formulations to prevent patient discomfort. There are two ways to calculate the adjustment we need: sodium chloride equivalence (SCE) and freezing point depression (FD). You can find SCE and FD1% values for common ingredients in the table at the end of this guide.
- Isotonic SCE = 0.9% sodium chloride (0.9 g NaCl per 100 ml water)
- Isotonic FD = 0.52 °C decrease in the freezing point of water
What is an Isotonic Solution?
Concentration and Osmotic Pressure
When we dissolve solids (a solute) in liquid (a solvent), we create a solvent with osmotic pressure, the pressure required to stop the movement of water across a membrane. Osmotic pressure is related to the solute concentration: the higher the concentration, the higher the osmotic pressure.
The primary solvent in biological systems is water, which can move between cells and the surrounding extracellular fluid through osmosis. The diagram below shows water moving from an area of low osmotic pressure to a region of high osmotic pressure through a semi-permeable membrane.

We can calculate the osmotic pressure of any solution by using the Van ‘t Hoff equation:
\Pi=i\left[ B \right]R\:T
- Π (upper case pi) = osmotic pressure
- i = osmotic coefficient constant, specific to each solute
- [B] = concentration of solute
- R = ideal gas constant
- T = temperature
The key takeaway is that the osmotic pressure of a solution doesn’t depend solely on the nature of the solutes! Varying concentrations [B] of different solutes (with different i values) can produce the same overall osmotic pressure, which will come in handy later.
Tonicity: Hypotonic, Isotonic and Hypertonic Solutions
Our cells are happiest when they contain the same osmotic pressure as the surrounding extracellular fluid—that is, they are in an isotonic environment. This means solvent movement in and out of the cell is at equilibrium, with no net flow.
However, if the surrounding fluid has a higher osmotic pressure (hypertonic), water inside the cell (with lower osmotic pressure) will move out, killing the cell by shrinking it (plasmolysis).
Water moves into the cell when the surrounding fluid has a lower osmotic pressure (hypotonic), causing it to swell and burst (cytolysis).
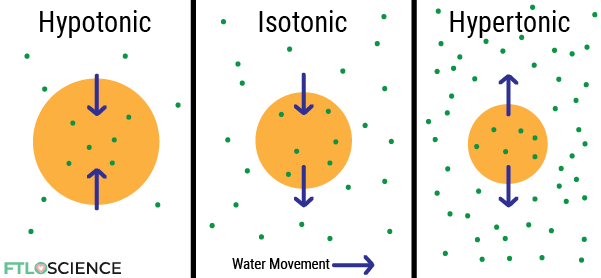
Why is an Isotonic Formulation Important?
Certain liquid medications like injections/infusions, eye drops and nasal rinses must be isotonic to prevent hypotonicity or hypertonicity in cells that they get in contact with.
Luckily, because osmotic pressure depends on the particles in a solution, we don’t have to use the exact chemical composition of our body fluids to create an isotonic solution!
As long as the osmotic pressure of a solution matches that of the inside of our cells, we can ensure there is no net flow of water that will cause plasmolysis or cytolysis.
Instead of using the Van ‘t Hoff equation, we will discuss two properties of solutes that allow us to calculate and adjust the tonicity of a solution quickly:
- The overall tonicity of body fluid is equal to a particular concentration of sodium chloride
- Dissolved particles in our body fluid decrease the freezing point of its solvent—water—by a specific value
Sodium Chloride Equivalent (SCE)
0.9% NaCl as a Reference Point
All the solutes in our body fluids contribute to an osmotic pressure that can be replicated with a 0.9% sodium chloride solution, or 0.9 g of NaCl in 100 ml of water.
We use NaCl as a reference point because it has an osmotic coefficient (i) of 1.8, which means that 1 molecule of NaCl produces around 1.8 particles (individual Na and Cl ions) when dissolved.
Most molecules have an osmotic coefficient of less than 1.8, meaning their tonicity contribution is less than the same amount of sodium chloride.
When taking molecular weights into account, we can express the tonicity contribution as a fraction of the same mass of sodium chloride. This is known as the sodium chloride equivalent (SCE); you can view the SCE of commonly used solutes in the table at the end of this tutorial.
Using SCE in Calculations
We often use several solutes in liquid formulations, all of which contribute to osmotic pressure. If the solution is hypertonic, we can dilute it, so it becomes isotonic. However, if the solution is hypotonic, we need to increase its osmotic pressure until it is isotonic, usually by adding sodium chloride.
How much sodium chloride do we add to a solution to make it isotonic? We know a 0.9% NaCl solution is isotonic, so we need to find the contribution from the rest of the ingredients using their SCE values.
For example, a 500 ml solution contains 5 g of boric acid and 10 g of sucrose. How many grams of NaCl do we need to make the solution isotonic? If we use glycerol instead, how many grams do we need?
| 1 | Determine the amount of NaCl to make 500 ml of pure water isotonic | 0.9 g NaCl in 100 ml gives an isotonic solution So for a 500 ml solution: (500/100)*0.9 = 4.5 g |
| 2 | Find the SCE values of the solutes (see table) | Boric acid = 0.52 Sucrose = 0.08 |
| 3 | Calculate the SCE contribution of the solutes | Boric acid = 0.52*5 = 2.6 Sucrose = 0.08*10 = 0.8 |
| 4 | Subtract the SCE contribution from the NaCl isotonic mass | 4.5 – 2.6 – 0.8 = 1.1 1.1 g of NaCl is needed to make the solution isotonic |
| 5 | Convert sodium chloride to glycerol by dividing by the SCE value | 1.1/0.35 = 3.14 3.14 g of glycerol can be used instead |
Freezing Point Depression (FD)
FD = 0.52 in Isotonic Solutions
The number of solute particles in a solution also affects its freezing point. The higher the osmotic pressure, the lower the solution’s freezing point. We call this freezing point depression (FD), caused by solute particles disrupting the bonds between solvent molecules.
It turns out that an isotonic solution of water causes it to have a freezing point of -0.52 °C; it has an FD of 0.52. We can use this information to adjust the concentrations of various solutes so that they give an FD of 0.52, making the solution isotonic.
Using FD1% in Calculations
Just like with SCE, we can calculate the FD contribution of several solutes in a solution and then adjust it to become isotonic by adding a certain amount of sodium chloride. We usually use FD1% values. the FD of water in a 1% solution (1 g in 100 ml) of a solute.
For example, a 200 ml solution contains 0.6% cetrimide and 1% propylene glycol. How many grams of NaCl do we need to make the solution isotonic?
| Step 1 | Find the FD1% values of the solutes (see table) | Cetrimide = 0.288 Propylene glycol = 0.262 |
| 2 | Calculate the solute FD contribution by multiplying % conc. with FD1% | Cetrimide = 0.288*0.6 = 0.173 Propylene glycol = 0.262*1 = 0.262 |
| 3 | Subtract the FD contribution from 0.52 (the FD of an isotonic solution) | 0.52 – 0.173 – 0.262 = 0.085 |
| 4 | Divide by the FD1% of sodium chloride to get the concentration of NaCl needed | 0.085/0.576 = 0.148 The sodium chloride conc. needed is 0.148% |
| 5 | Find the mass of NaCl needed for a 200 ml solution | 0.148% means 0.148 g per 100 ml So for a 200 ml solution: (200/100)*0.148 = 0.296 0.296 g of NaCl is needed to make the solution isotonic |
SCE and FD1% of Common Solutes in Pharmaceutical Formulations
Table of common chemicals in liquid formulations, with sodium chloride equivalent and freezing point depression 1% values. Values from Australian Pharmaceutical Formulary Handbook (APF21).
| Chemical | SCE Values (g) | FD1% Values (°C) |
|---|---|---|
| Acetic acid | 0.54 | 0.31 |
| Ammonium chloride | 1.08 | 0.64 |
| Ascorbic acid | 0.18 | 0.105 |
| Atropine sulfate | 0.13 | 0.074 |
| Benzalkonium chloride | 0.16 | 0.09 |
| Boric acid | 0.52 | 0.288 |
| Cetrimide | 0.09 | 0.05 |
| Chloramphenicol | 0.14 | 0.078 |
| Chlorbutol | 0.24 | 0.14 |
| Citric acid monohydrate | 0.18 | 0.098 |
| Cocaine hydrochloride | 0.16 | 0.09 |
| Dextrose monohydrate | 0.16 | 0.09 |
| Ephedrine hydrochloride | 0.29 | 0.165 |
| Epinephrine hydrochloride | 0.29 | 0.17 |
| Ethanol | 0.7 | 0.41 |
| Glycerol | 0.35 | 0.203 |
| Lactose | 0.07 | 0.04 |
| Mannitol | 0.17 | 0.098 |
| Morphine hydrochloride | 0.15 | 0.086 |
| Neomycin sulfate | 0.11 | 0.063 |
| Phenylephrine hydrochloride | 0.32 | 0.185 |
| Pilocarpine hydrochloride | 0.24 | 0.138 |
| Potassium hydrochloride | 0.76 | 0.439 |
| Propranolol | 0.20 | 0.122 |
| Propylene glycol | 0.45 | 0.262 |
| Silver nitrate | 0.33 | 0.19 |
| Sodium bicarbonate | 0.66 | 0.38 |
| Sodium chloride | 1.00 | 0.576 |
| Sodium sulfate | 0.26 | 0.148 |
| Sucrose | 0.08 | 0.047 |
| Sulfacetamide sodium | 0.23 | 0.132 |
| Tetracycline hydrochloride | 0.14 | 0.081 |
| Zinc chloride | 0.59 | 0.35 |
| Zinc sulfate | 0.15 | 0.086 |
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




