Halloween is just around the corner! The season of jack-o’-lanterns, trick-or-treating and good scares also heralds the return of pumpkin spice. We find excuses to use this uniquely delicious blend of spices in lattes, pies and even salad dressing. But did you know that pumpkin spice doesn’t contain any pumpkin at all? We break down the ingredients and chemicals that make up this holiday seasoning.
Pumpkin spice doesn’t contain any part of the pumpkin as an ingredient. Instead, most recipes list cinnamon, ginger, nutmeg, clove and allspice. The unique chemical in each, as well as their primary effects, are shown in the table below:
| Ingredient | Unique Chemical | Primary Effects |
|---|---|---|
| Cinnamon | Cinnamaldehyde | Cinnamon flavor; insecticide and mosquito repellent |
| Ginger | Gingerol | Activates spice receptors; may help diabetics regulate glucose |
| Nutmeg | Myristicin | Hallucinogen; is a precursor to amphetamines |
| Clove and Allspice | Eugenol | Spicy flavor; is an antiseptic and anesthetic |
Ingredients and Chemicals in Pumpkin Spice
Cinnamon and Cinnamaldehyde

Cinnamon is actually the bark of cinnamon trees. Rolled up within the bark is the chemical cinnamaldehyde, which gives the spice its distinct, pungent scent. In fact, some powdered ‘cinnamon’ you find in supermarkets don’t contain cinnamon at all! They are just ground powders of a tasteless substance with cinnamaldehyde added.
Cinnamaldehyde is also effective as an insecticide, either mixed in water to kill mosquito larvae or as a gaseous fumigant. Some studies show that it can also kill bacterial and fungal cells, making it useful as an antimicrobial agent.
There is evidence that cinnamaldehyde protects our cells against DNA damage, reducing the chance of random mutation appearing. However, this doesn’t make it an anticancer agent, so be wary of people who claim that eating cinnamon prevents cancer!
Ginger and Gingerol
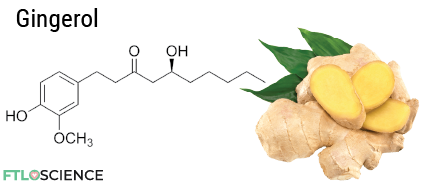
Gingerol is what makes ginger spicy (spicier than tabasco but not as spicy as habanero). Did you know that cooking ginger reduces its spiciness? This is because high temperatures convert its main ingredient, gingerol, into zingerone—where KFC’s Zinger burger gets its name.
Eating ginger prevents diarrhea by blocking fluid accumulation in the small intestine. In obese and type II diabetic mice, ginger in their diet helped increase glucose uptake despite their lack of working insulin.
Gingerol has been shown to prevent the growth of cancer cells by interfering with mitochondrial proteins, though it also causes DNA damage to human cells at higher concentrations.
It’s important to note that the results of these studies only apply in vitro (in a test tube) and not on animals and humans. Since no clinical trials with gingerol have yet been done, we can’t ensure its safety and effectiveness as a treatment.
Nutmeg and Myristicin
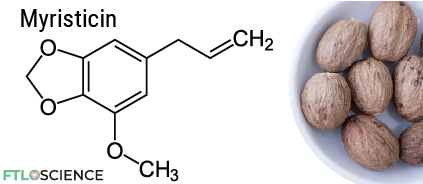
Although myristicin isn’t the most abundant chemical in nutmeg, it is arguably the most exciting! You see, eating too much nutmeg can cause hallucinations, thanks to the psychoactive effects of myristicin.
However, eating more than three nutmegs (or 15 g of nutmeg spice) contains enough myristicin to cause poisoning, which can lead to serious neurological effects and death. There is currently no antidote for myristicin poisoning.
Myristicin is closely related in chemical structure to methamphetamine and amphetamine. It can be used to synthesize MDMA (ecstasy) and other related compounds, shown below. In our livers, CYP450 enzymes break down myristicin into MMDA, the likely source of its brain-altering effects.
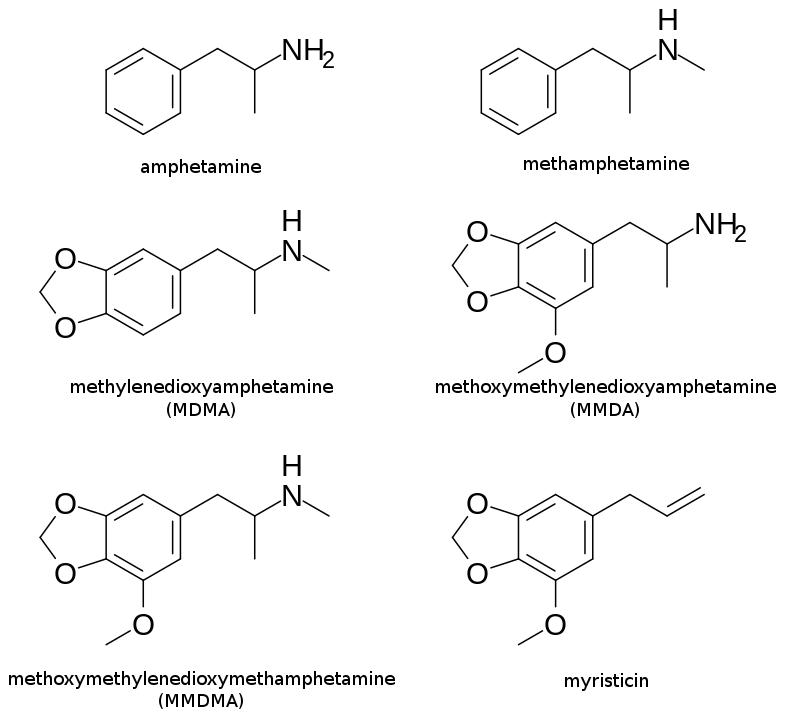
Due to its psychoactive effects, it has been studied for use as a therapeutic for central nervous system disorders, but nothing conclusive has come out of it. Myristicin might also be useful as a chemo-protecting agent, helping to ease the side effects of certain chemotherapies.
Clove/Allspice and Eugenol

Clove and allspice are, respectively, the flower buds and fruits of trees in the plant family Myrtaceae (myrtle). The main component of both is eugenol, a chemical that has a distinctive spicy scent and is a favorite for many teas, perfumes and essential oils.
Eugenol has antiseptic properties, killing many species of bacteria, including Staphylococcus aureus and Escherichia coli. Being an alcohol molecule, eugenol has a reactive hydroxyl group (-OH) in its structure that allows it to damage the cell membrane of bacteria.
We’ve been using eugenol for oral and dental care since ancient times to disinfect our mouths and fill cavities. Today, it’s also used as an anesthetic in dentistry to numb and relieve pain from toothaches and dentures.
A Generous Dash of Skepticism
There you have it: the real ingredients in pumpkin spice with a dash of facts! However, while looking at each chemical in pumpkin spice is interesting, it’s important to remember that none of these compounds have been shown to work as a therapy for any illness whatsoever.
Furthermore, many plant sources have high levels of toxic substances, especially when concentrated in essential oils (I’m looking at you, aromatherapy). So don’t assume something is safe just because it comes from a plant.
We hope you’ve enjoyed this Halloween special. Now that you’ve learned about the chemistry of pumpkin spice, you can share the treat of knowledge with the kids who come knocking on your door. Happy Halloween!
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




