Merry Christmas! As we relax and unwind this holiday season, look out the window to observe the crystals that form on the pane. Do you realize that each snowflake has six sides, regardless of its shape or size? Each icy crystal begins its existence high up in the atmosphere as a single mote of dust, slowly altering its appearance into a delicate snowflake as it falls to the ground. Throughout this process, snowflakes always retain their six-sided, hexagonal structure, nature ever the master craftsman.
From Water Vapor to Beautiful Snowflake
How Snowflakes Form
Like rain, snowflakes are a form of precipitation, where water molecules high up in the atmosphere return to the surface of the Earth. Unlike rain, however, snowflakes fall to the ground gently, mesmerizing us with its six-sided beauty.
The birth of each snowflake can be attributed to a nucleation event. A particle such as a speck of dust comes into contact with cold water vapor in the atmosphere and allows water molecules to freeze on its surface. The snowflake can now begin its growth in earnest.
As they get heavier, the bound molecules are pulled downward by gravity. As the snowflake falls, it maintains a fine balance between the rate of water molecules diffusing toward the ever-growing flake and the rate of crystal formation at its interface, causing it to grow into beautiful six-sided shapes.
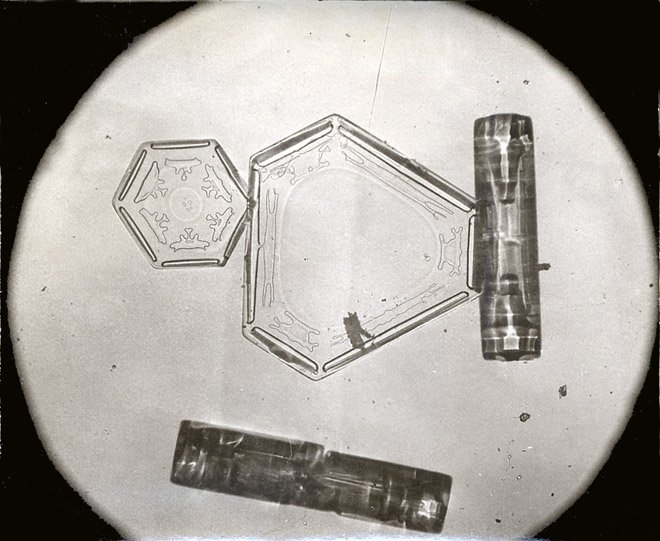
Unique Snowflakes
A higher rate of water diffusion drives the snowflake toward irregular growth, maximizing its surface area. A higher rate of crystal formation competes by cultivating the growth of longer patterns and overall symmetry. These rates change with temperature and humidity (saturation range), resulting in snowflakes of different shapes, but always retaining their six-sided geometry.
The minuscule differences in the environment and conditions that each individual snowflakes experience as they fall give each a unique pattern, from needles and laces to the most complicated and wonderfully crafted prisms and plates. This also explains the chances of improbability of finding two identical snowflakes in nature!
This table shows a general classification of snowflakes that form at different temperatures and humidity levels (saturation ranges)1:
| Temperature Range | Saturation Range (g/m3) | Types of Snowflakes Below Saturation | Types of Snowflakes Above Saturation |
|---|---|---|---|
| 0°C (32°F) to 3.5°C (26°F) | 0.0 to 0.5 | Solid plates | Thin plates Dendrites |
| 3.5°C (26°F) to 10°C (14°F) | 0.5 to 1.2 | Solid prisms Hollow prisms | Hollow prisms Needles |
| 10°C (14°F) to 22°C (8°F) | 1.2 to 1.2 | Thin plates Solid plates | Sectored plates Dendrites |
| 22°C (8°F) to 40°C (40°F) | 0.0 to 0.4 | Thin plates Solid plates | Columns Prisms |
Why Do All Snowflakes Have Six Sides?
Six-Sided Symmetry
Some people would have learned, or realized, that every snowflake has six sides, regardless of its shape or size. If you didn’t know that already, how cool is that?
This phenomenon was first explained by the German astronomer and mathematician Johannes Kepler in 1611, in which he stated that the reason for a snowflake having six sides would not be found in the material’s characteristics, since water vapor is formless, but in an unseen agent.
He meant that this special agent worked through more mechanical means, using the smallest natural unit of water used as a template to align and stack subsequent building blocks. In other words, the solution to this question could be found by looking at the water molecule itself.
With the help of fellow mathematician Thomas Harriot—who had a thing for packing cannonballs in close quarters—Kepler asserted that hexagonal packing would be the tightest and most efficient, such that in no other arrangement could more pellets be stuffed into the same container.
This assertion about maximal close-packing is known as the Kepler conjecture and also gave rise to a whole area of study of crystal and lattice chemistry which we have all come to know and love.

Water Molecule Geometry in Six-Sidedness
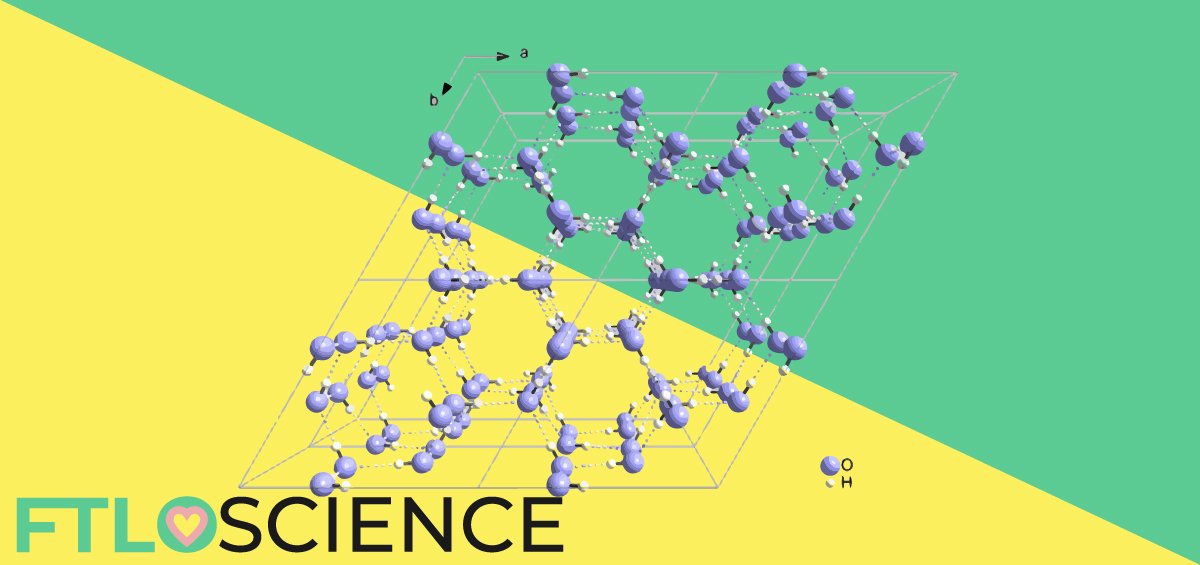
From the diagrams below, it is easy to picture how the hydrogen atoms in each water molecule interact with the two lone pairs of oxygen to form a six-sided structure through hexagonal close packing. In this structure, water molecules in ice are forced into adopting a wider opening angle (106.6° rather than the 104.5° observed in ‘free’ water).
This means that every connecting point is facing either directly on top or below the trigonal plane (you can see this on the diagram on the right clearly, where alternate black spheres have connecting points facing upward). This allows for the connection of open, hexagonal rings that make ice less dense than water, also explaining the six-sided geometry of a snowflake!

We now know that Thomas Harriot was correct; water packs in a hexagonal close-packed structure. But it isn’t due to the shape of the water molecule. Just like with cannonballs, a range of different close packing structures is possible. It just happens that temperatures in our atmosphere causes water to favor the adoption of ‘hexagonal ice’. Under different conditions, water can also form ‘cubic ice‘ or ‘trigonal ice’, with eight and three sides, respectively.
So we’ve uncovered the reason for all snowflakes having six sides. But why then, if given the opportunity to expand in all directions, do snowflakes grow so thin? Why do their corners grow many thousand times faster than the top and bottom faces?
Apparently, this is a phenomenon that only occurs in the narrow temperature range that is our atmosphere. Ice formation takes the shape of larger and bulkier prisms in other temperatures! Maybe one day, when traveling through space to a more frigid planet, we might bring home snowprisms as souvenirs.
Thank you once again for taking the time to read this, we hope you enjoy it and we would also like to take this opportunity to wish all our readers a very merry festive period. Enjoy your holidays, wherever you may be, and count yourselves lucky if you are able to observe snowflakes in all their six-sided glory.

Reference
- Bishop, M. P., Bjo¨rnsson, H., Haeberli, W., Oerlemans, J., Shroder, J. F., & Tranter, M. (2011). Encyclopedia of snow, ice and glaciers. Springer Science & Business Media.
- Ben-Jacob, E., & Levine, H. (2001). The artistry of nature. Nature, 409(6823), 985-986.
- Ball, P. (2011). In retrospect: on the six-cornered snowflake. Nature, 480(7378), 455-455.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.