Throughout history, diethylene glycol poisoning has been a regular occurrence, with fatalities from consuming the chemical arising once every few years. Despite our knowledge of the dangers of diethylene glycol (DEG), why does this chemical keep killing us? In this article, we will explain the sources of DEG poisoning and compare the toxicities between members of the glycol class of compounds.
Ethylene glycol and diethylene glycol belong to a group of chemicals known as glycols: viscous, sweet-tasting liquids. They are metabolized into toxic byproducts by enzymes in our liver, namely alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Glycol poisoning is a common occurrence because they are easily mistaken for syrup during liquid drug product manufacture.
Why is Diethylene Glycol Poisoning So Common?
The Poison that Keeps on Killing
There have been many high-profile cases of diethylene glycol (DEG) poisoning over the years; we’ve highlighted several below:
- The first recorded case of DEG occurred almost 100 years ago, in 1937. Massengill, one of the first pharmaceutical companies established in the U.S., produced sulfanilamide (an antibiotic) using DEG as a solvent, leading to over 100 deaths in 15 states.
- In 1986, doctors in a hospital in India prescribed glycerol (glycerin) to patients as a diuretic, but 21 of those died from kidney failure. It turns out that the glycerol solution contained a high percentage of DEG.
- A major scandal in 1995 involved manufacturers in China supplying DEG-contaminated syrup barrels to factories producing acetaminophen (Tylenol/Panadol) in Haiti, which led to at least 88 child deaths in the country.
- The most recent high-profile case of DEG poisoning occurred in 2022 in Gambia and Indonesia. Both countries reported deaths (mostly children) after taking cough syrups contaminated with ethylene and diethylene glycol, linked to Maiden Pharmaceuticals in India. At the time of writing, investigations into this spate of poisonings are still ongoing.
The question is: after almost a century of DEG poisonings, why haven’t we been able to stop them from occurring at all?
Usefulness of DEG in Manufacturing Industries
Diethylene glycol (DEG) is widely used in manufacturing because of several interesting properties:
- DEG can dissolve chemicals that are insoluble in water
- It is a viscous liquid even at sub-zero temperatures
- It is colorless and odorless (though it has a slightly sweet taste)
- It is cheap to produce.
Products that contain DEG include:
- Antifreeze formulations and brake fluids (thanks to its low freezing point)
- General lubricant solutions
- Artificial fog/smoke machines (it forms an opaque haze when vaporized)
- Cosmetics (it is a humectant)
- Chemical reagents (DEG is used as a solvent)
Contamination in Pharmaceutical Products
A critical source of DEG contamination is drug products, namely liquid medications. In pharmaceutical formulations, drug compounds can be poorly water-soluble, so manufacturers add glycerol or propylene glycol—non-toxic, viscous substances—to improve their solubility. They also have the advantage of being slightly sweet, helping to mask the bitter taste of many drug chemicals.
The problem is that glycerol and propylene glycol are similar in chemical structure and are visibly identical to diethylene glycol. There have been instances in which suppliers intentionally swap glycerol for diethylene glycol (a cheaper chemical) to sell as counterfeit reagents, or simply mistake the chemicals for one another. To compound this problem, its sweet taste appeals to children who might get their hands on DEG-contaminated products.
Ethylene Glycol vs. Diethylene Glycol: What’s the Difference?
Glycol Class of Compounds
Ethylene glycol and diethylene glycol are members of a larger group of chemicals known as glycols. Glycols are organic compounds that contain two alcohol (-OH) groups in their chemical structure. They are also known as diols since they contain two (di) alcohols.
The simplest glycol is ethylene glycol (EG) with a chemical formula of (CH2OH)2. EG is produced from carbon monoxide, hydrogen and oxygen. During its production, diethylene glycol and triethylene glycol are also formed as byproducts.
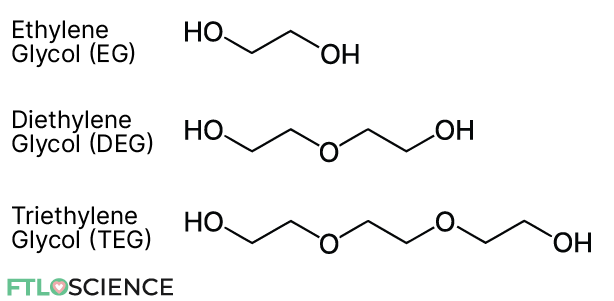
Other related compounds include propylene glycol (PEG) and glycerol, though they are produced differently. Note that glycerol is not considered a glycol since it has three alcohol groups (if you look closely, it’s just PEG with an additional -OH group slapped on).

Different Mechanisms of Action
In this section, we’ll focus on the toxicities of ethylene glycol (EG) and diethylene glycol (DEG) since they comprise the bulk of glycol poisoning cases. Though we know that their toxicities come about through different mechanisms of action, neither is particularly well understood.
Both EG and DEG can cross the blood-brain barrier in their original forms, causing neurological effects that mirror alcohol poisoning: intoxication, depression, headaches and, in severe cases, seizures and vomiting.
Subsequently, EG is broken down in the liver by an enzyme called alcohol dehydrogenase (ADH), producing a variety of chemicals, including glycolaldehyde, glycolic acid and oxalic acid. Oxalic acid is converted into its salt, calcium oxalate, which makes its way into the kidneys and forms crystals. This can lead to kidney failure and death if not treated quickly.
DEG is also broken down in the liver by ADH and its related enzyme aldehyde dehydrogenase (ALDH), producing (2-hydroxyethoxy)acetaldehyde and (2-hydroxyethoxy)acetic acid (HEAA).
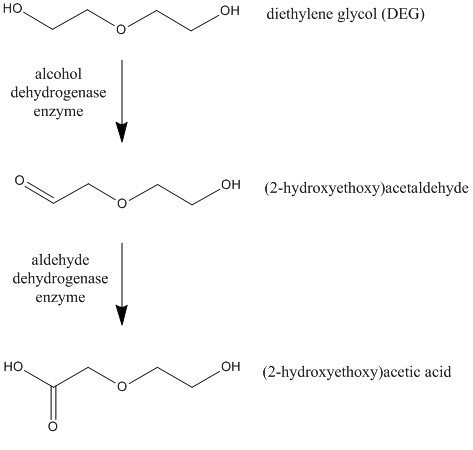
DEG poisoning also leads to acute kidney failure in patients, but not through oxalic acid and calcium oxalate. Instead, HEAA has been hypothesized as the chemical culprit in these cases, although its mechanism of action isn’t certain.
Research shows that HEAA might cause cellular toxicity that leads to acidosis (decreasing blood pH), while HEAA and the DEG molecule itself can cause kidney and liver failure.
Comparing Toxicities of Glycol Compounds
The table below shows LD50 (the dose that kills half a population) of different glycol points in rats.
| Chemical | Oral LD50 (Rat) |
|---|---|
| Ethylene Glycol (EG) | 7.7 g/kg |
| Diethylene Glycol (DEG) | 12.6 g/kg |
| Triethylene Glycol (TEG) | 19.4 g/kg |
| Propylene Glycol (PEG) | 20 g/kg |
It is important to note that though human toxicities have not been well studied, humans seem to be more sensitive to glycol poisoning than rats. For example, 30 ml of ethylene glycol has caused death in an adult, translating to just 0.47 g/kg (in an average 70 kg adult), more than 10 times smaller than the LD50 value of 7.7 g/kg in rats. DEG also shares this characteristic.
The first-line (standard) treatment for glycol poisoning is to block the liver enzyme responsible for breaking it down—alcohol dehydrogenase (ADH). As with methanol poisoning, the ADH inhibitor fomepizole is an effective treatment, while ethanol (alcohol) also works in a pinch.
When available, hemodialysis (blood replacement) is also administered because once EG and DEG are metabolized, the byproducts entering the bloodstream rapidly cause kidney failure and death.
Controlling Glycol Usage
While glycols are useful products, their toxicities have led to controls and replacements being put in place. Products like antifreeze solutions are substituting EG with less toxic glycols like PEG in their ingredients list. Other control methods include adding bitter flavorings to EG and DEG-containing products, deterring their ingestion (both intentional and unintentional).
There are strict limits on the amount of DEG that can be used in products sold in the U.S., while other countries like Australia have banned DEG as a chemical additive altogether. However, continued cases of DEG poisoning suggest that more needs to be done to improve safe practices in the supply and manufacture of glycol products.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




