Did you know that detergents are often added to gasoline? While they might not be identical to the ones used in laundry and dishwashing solutions, gasoline detergents help our car engines stay clean and free from combustion byproducts. We discuss the different types of additives and the chemistry of detergents in gasoline in this article.
Detergents are surfactants, chemicals that contain both polar and non-polar groups in their molecular structure. Their dual nature helps break down insoluble solids that could block and cause damage to our car engines. Common gasoline detergents include amine-based, alcohol-based (ethanol/methanol), sulfur-based and zinc-based molecules.
Gasoline and Combustion Chemistry
What Goes on in Car Engines?
Car engines work through the controlled combustion of gasoline—a combination of hydrocarbon molecules of different lengths. The heat and pressure from this combustion powers a piston that rotates the wheels of a car.
The efficiency of gasoline combustion depends mainly on the mixture of hydrocarbons, giving rise to different gasoline ratings. In car engines, branched hydrocarbons tend to burn more efficiently than linear ones because they are more chemically stable.
Additives Found in Gasoline
Although hydrocarbons from crude oil distillation make up the bulk of gasoline, a small amount of additives are found in gasoline that helps improve its efficiency in car engines. These additives include:
- Stabilizers that improve the chemical stability of the gasoline mixture, ensuring it stays fresh and preventing chemical reactions that break down the hydrocarbons. These are mainly phenol derivatives (butylated hydroxytoluene, butylated phenols) and diamines (ethylene diamine).
- Corrosion inhibitors are also added to gasoline to prevent harsh chemicals from damaging the engine mechanism. Oxygen is the main culprit, able to oxidize and corrode metal parts in the engine. To combat this, hydrazine and other amines can be added to convert oxygen to water. Other antioxidants like zinc dithiophosphates are commonly used in fuel mixtures as corrosion inhibitors.
- Antiknock agents that prevent premature combustion (‘engine knock’), which can lower the efficiency of the engine and cause damage in the long run. Examples: toluene, isooctane and other branched hydrocarbons.
- Detergents to keep the engine clean across many combustion cycles, preventing the build-up of gasoline combustion byproducts on the engine mechanism (especially the fuel injector, pistons and outlet).
- Other less common additives include dyes and fuel enhancers like nitromethane (‘nitro’ in racing fuel) and ether to help engines start.
The Role of Detergents in Gasoline
Byproducts of Incomplete Combustion
Detergents, as an additive in gasoline, help keep its combustion clean. While detergents cannot help keep the environment clean (they don’t reduce emissions), they ensure your car engine is free from dirt buildup and combustion byproducts. But where do these byproducts come from?
The only byproducts in 100% efficient combustion should be heat, water and carbon dioxide:
\text{C}_{x}\text{H}_{y} + \text{O}_{2}\to \text{H}_{2}\text{O}+\text{CO}_{2}These chemicals do not pose a problem to car engines. However, no reaction is fully efficient, and—especially in lower-rated gasoline mixtures—incomplete combustion is common:
\text{C}_{x}\text{H}_{y} + \text{O}_{2}\to \text{H}_{2}\text{O}+\text{CO}_{2}+\text{CO}+\text{C}While carbon monoxide (CO) isn’t too big of an issue (to the car engine anyway), the excess carbon takes the form of sludge and soot that sticks to the engine’s insides instead of being flushed away by the combustion cycle. This reduces the engine’s power and efficiency and can damage it over time.
How Detergents Work to Remove Engine Contaminants
Detergents (also known as surfactants) are chemicals that have a polar (water-soluble) head group and a non-polar (oil-soluble) tail. This is useful as they can interact with both polar and non-polar compounds.
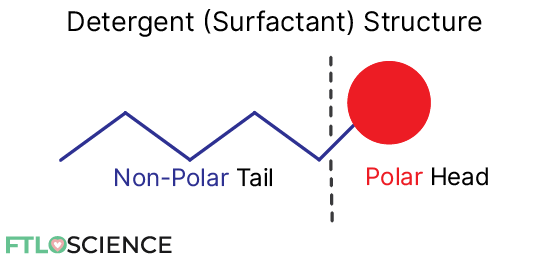
This dual nature enables detergents to solubilize both water-based and oil-based contaminants that might get stuck in the engine. Apart from combustion byproducts, dirt and grime can find their way into the engine and get stuck, especially around the fuel injectors and pistons.
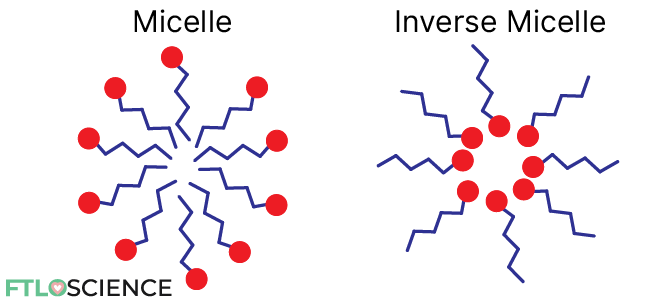
Detergents can form spheres known as micelles by aligning their polar head group on the outside, with their non-polar tails facing inward. This creates a central space that can trap and solubilize non-polar substances.
By reversing this alignment (non-polar tails facing outward and polar heads inward), detergents can also create a polar cavity, which is useful for dissolving water-based substances that would otherwise be insoluble in hydrocarbon-rich gasoline.
Detergent Composition in Gasoline Mixtures
By far the most common detergents in gasoline are amine-based detergents, including polybuteneamine (PBA), polyetheramine (PEA) and polyisobutyleneamine (PIBA). Amine-based detergents have an amine (nitrogen-containing) head group and a hydrocarbon tail (or tails).
However, gasoline brands treat the actual chemical composition of their mixtures as a trade secret, so the exact variety of detergents used in gasoline isn’t known to the public. Alcohol-based, sulfur-based and zinc-based detergents in gasoline have been characterized.
Since gasoline producers use a range of proprietary blends in their mixtures, in 2004 a group of automakers established guidelines to test and regulate gasoline for their detergent effectiveness.
These tests include measuring valve and combustion chamber deposit buildup over the course of many cycles, as well as performance tests to ensure the overall proper function of the car engine. This set of standards is labeled TOP TIER, and only gasoline brands that can meet these standards are given the license to market it as such.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




