With crude oil prices reaching record highs earlier this year, it’s no surprise that we want to get the most mileage from our gas (petrol). Our car engines run on gasoline, a mixture of carbon-rich alkanes with different lengths known as hydrocarbons. It turns out that branched hydrocarbons run more smoothly than linear (straight) ones. In this article, we look at the chemistry of combustion!
Gasoline is a mixture of both branched and linear (straight) hydrocarbons. Car engines compress gasoline with air at high pressures, causing reactive free radicals to form. Branched hydrocarbons burn better in car engines because they are more stable, providing controlled combustion. The more reactive linear hydrocarbons often undergo premature combustion, commonly known as engine knock. This decreases the engine’s efficiency and can damage the piston over time.
What is Gasoline?
Drilling for Crude Oil
Gasoline (petrol) is produced from separating crude oil (petroleum), which we extract by drilling into reservoirs deep within the ground or sea. Crude oil forms when carbon-rich remains of animals and plants are subject to high pressures over many years. 66 million years, to be exact. Humans as a species have only been around for 300,000 years, less than 1% of that time!
Oil refineries then separate crude oil into a range of valuable products, including gasoline. They use fractional distillation, a highly efficient process—only 15% of crude oil is lost as waste.
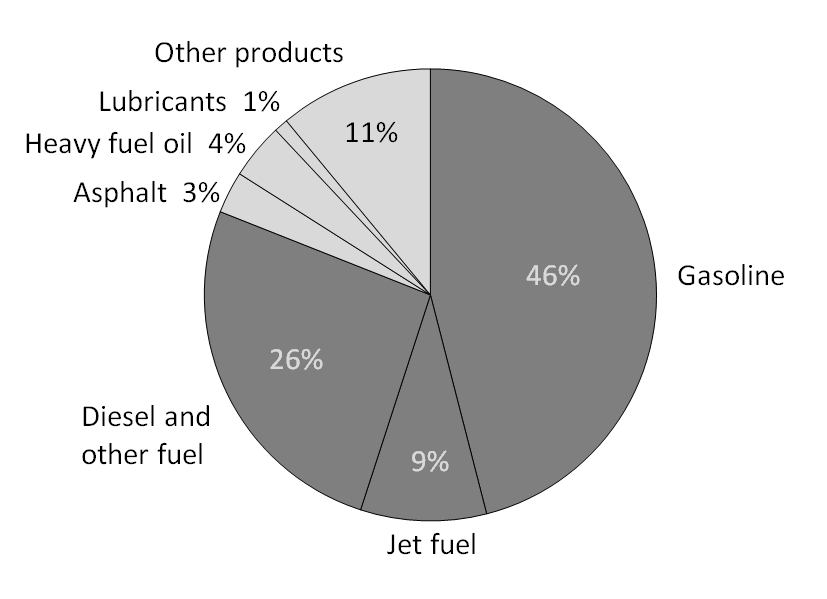
Chemical Composition of Gasoline
Gasoline is a mixture of hydrocarbons, mainly alkanes, with between 4 and 12 carbon atoms. Some of these have branched chemical structures, while some are straight/linear (shown below).
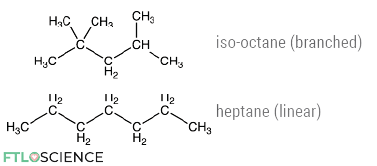
The proportion of branched vs. linear hydrocarbons in a gasoline mixture affects its efficiency in car engines, represented by its research octane number (the rating shown on gas pumps).
The Chemistry in Car Engines
It’s Called Deflagration, Not an Explosion
Gasoline burns in the presence of a spark at temperatures above −23 °C (−9 °F), its flash point. Car engines take advantage of this flammability, injecting gasoline and air into a chamber and igniting the mixture with a spark plug.
This ignition causes deflagration, a rapid increase in heat and pressure that expands the volume within the chamber. This pushes a piston downward , powering the rotation of the car’s wheels. The piston then rotates upward, removing the spent mixture and restarting the cycle. Depending on the engine, there are between 4 and 16 such pistons powering the car.
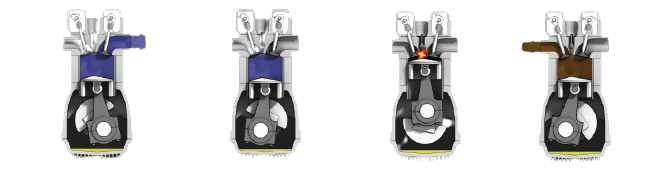
Ignition should occur only when the gasoline/air mixture is fully compressed (3rd step in the image above). Sometimes, the mixture combusts before it this happens, disrupting the cycle and reducing the efficiency of the engine. This is known as engine knock; if you want to hear it, here’s a video.
All About Free Radicals
It turns out that branched hydrocarbons behave well in car engines, igniting when it’s supposed to at the right moment in the cycle. Linear hydrocarbons, on the other hand, are prone to prematurely combusting midway through the compression stage, causing engine knock.
This is due to the differences in their reactivity. You see, compressing hydrocarbons in the presence of air causes them to behave strangely. They can lose a hydrogen atom, forming highly reactive free radicals.
These free radicals can kick start a chain reaction, reacting with oxygen in the fuel/air mixture and creating even more radicals. This quickly results in an uncontrollable mini-explosion, which is not ideal!
Branched Vs. Linear Hydrocarbons
In chemistry, free radicals are molecules that have an unpaired electron. Electrons love being in pairs, so free radicals are always looking out for other molecules to react with, grabbing their electron in the process.
Branched hydrocarbons are less reactive because their branched structure supports this unpaired electron, like a buddy who holds back their unstable friends from getting into fights.
The long and thin structure of linear hydrocarbons doesn’t provide this support, allowing the unpaired electron to react with oxygen in the mixture. This starts the chain reaction that ends in premature combustion and causes engine knock.
Other Factors that Affect Combustion
It’s not just branching that affects the behavior of the gasoline mixture! Hydrocarbon chain length also changes its reactivity, with studies showing that longer linear alkanes are more reactive than shorter ones. The graph below shows that the ignition delay decreases as the hydrocarbon chain length grows.
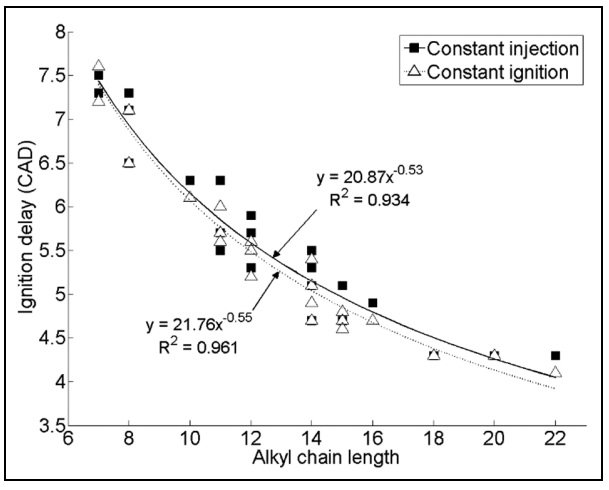
Having more double bonds and adding oxygen atoms also makes the mixture more stable, increasing the ignition delay. These chemical factors are critical when formulating gasoline mixtures that burn efficiently in car engines and reduce engine knock.
Improving Efficiency of Gasoline
We all know that the rate at which we’re drilling for oil isn’t sustainable, with worldwide reserves likely to run out before the end of the century. Furthermore, emissions from burning fossil fuels contribute to pollution and climate change. The problem is that gasoline as a fuel is so good at getting us from A to B.
Despite the recent rise in the popularity of electric cars, gasoline-powered vehicles still dominate—holding 90% of the market share. Alternatives like biofuel (ethanol) exist, but our current production methods aren’t nearly as efficient as extracting gasoline from crude oil.
Ethanol is commonly added to gasoline mixtures (E10, E20, etc. at the gas pump), usable in most engines.
While we wait for a viable alternative to gasoline, our best bet is to continue to improve gasoline mixtures, using branched hydrocarbons and long-chain alkanes so that engines run smoothly, efficiently and with low emissions.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.



