You drive into a gas (petrol) station and fill your car’s tank with 91-rated fuel—unleaded, of course. There’s no way you’re touching that E10 or E20, they’re dodgy and will mess with your engine! Do you know what makes up the gasoline that powers your ride? Are you curious to know if ethanol will actually work with your engine? Well, stop wondering as we break down the different petrol ratings, and how their chemistry affects the performance of your car!
Gasoline mixtures are rated before being sold—a higher rating means the mixture burns more efficiently in car engines. We can improve this rating by increasing the amount of octane in the mixture. Ethanol can also be mixed with gasoline to increase its efficiency, but the tradeoff is a decrease in energy output. An E-rating in gasoline tells us the % of ethanol within:
- E10 = 10% ethanol mixed with 90% 91-rated gasoline
- E20 = 20% ethanol mixed with 80% 91-rated gasoline
- etc.
Studies have shown that most car engines can run on up to E20 (20% ethanol) gasoline without any long term issues.
Car Engine Basics
Igniting Gasoline Turns Your Wheels
Gasoline (petroleum) is made up of hydrocarbons: long-chain molecules consisting mainly of carbons and hydrogens. These molecules are highly efficient in storing energy, which is why they are a top choice for powering our rides. Of course, fossil fuels being a non-renewable resource is a growing concern, but alternatives like biofuels are still a long way from replacing gasoline in cars.
Car engines work by injecting a mixture of fuel and air through the top of a hollow cylinder, which is then compressed by a piston. When the optimal pressure is reached, the fuel/air mixture is ignited by a spark plug. This causes causing an explosion, with the air pushing the piston outward and powering a crankshaft. The crankshaft rotates, causing your wheel to turn.
In a car, there are normally 4 such pistons (though some engines have up to 16). The pistons are ignited at different times, keeping your wheels turning smoothly. If there were just one large piston, your car would sputter along rather nicely.
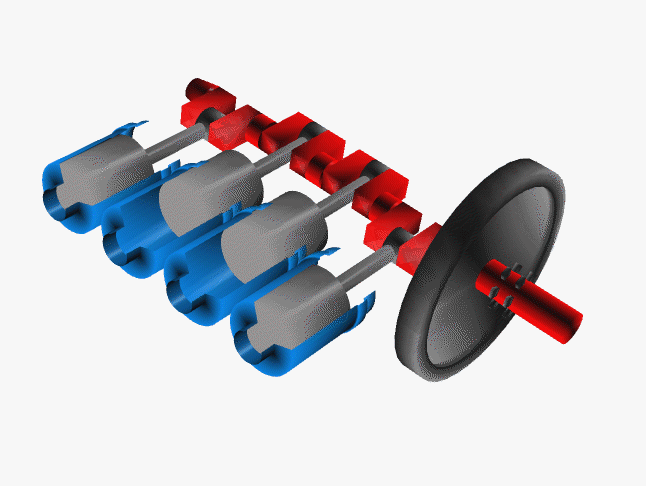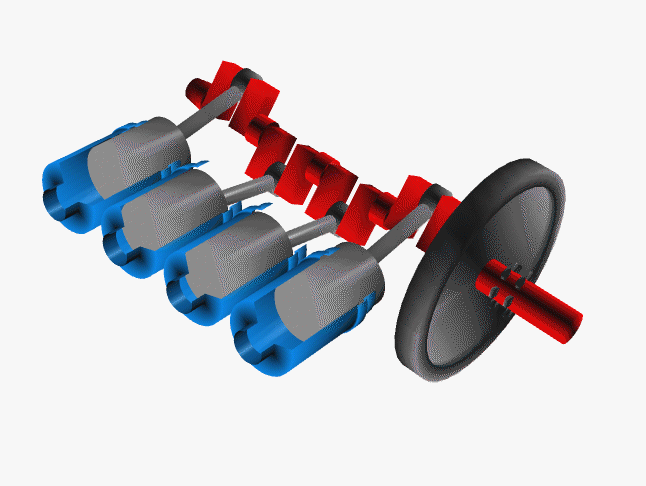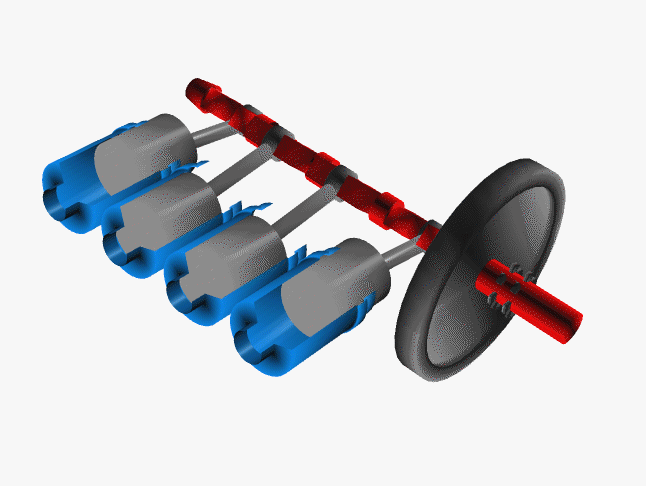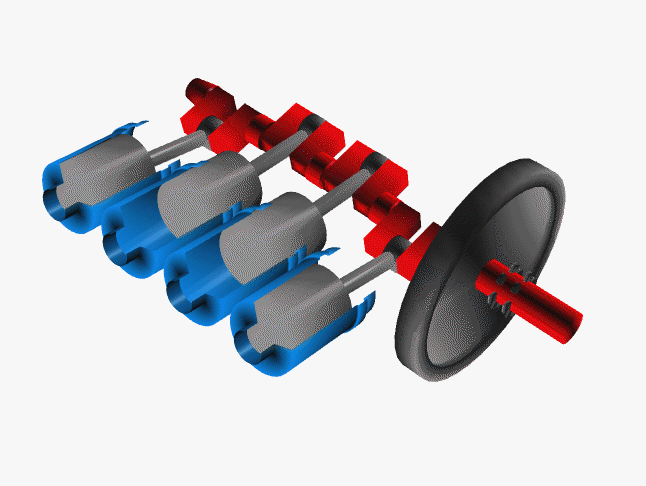
The Chemistry of Engine Knocking
Due to high pressures and heat in the engine, sometimes the fuel/air mixture autoignites prematurely before the spark plug can do its job. This is known as engine knocking. Engine knock is bad because it releases so much energy, which can damage the pistons over time. So how can we reduce engine knock?
Gasoline is made up of a range of hydrocarbons (between 4 and 12 carbons in length), as well as some additives. Branched hydrocarbons happen to burn more efficiently than linear hydrocarbons.
The likelihood of premature autoignition of fuel is reduced by increasing the number of branched hydrocarbons (like iso-octane). Long-chain hydrocarbons (like heptane) are prone to engine knock. This is why gasoline mixtures have different efficiencies (and prices!)

Bottom: heptane
Making Gasoline More Efficient
Altering the Composition of Gasoline Mixtures
All petrol that is sold at gas station must undergo a test to determine its rating. It’s used to power an engine and the amount of engine knocking is measured. Then value is then compared to reference fuels with known mixtures of iso-octane and heptane. The amount of engine knock is higher for mixtures that have a smaller percentage of iso-octane.
| Gasoline RON | Mixture | Engine Knock |
|---|---|---|
| 91 | 91% iso-octane 9% heptane | Highest |
| 93 | 93% iso-octane 7% heptane | High |
| 95 | 95% iso-octane 5% heptane | Low |
| 100 | 100% iso-octane | Lowest |
The ratio of iso-octane and heptane is represented by its Research Octane Number (RON 91, 93, 95, etc.), with a higher number meaning a larger proportion of iso-octane, which in turn means less engine knock.
For example, gasoline that is tested to have the same amount of engine knock as a test mixture of 91% iso-octane and 9% heptane would be given a RON of 91. Some additives are more efficient than iso-octane, which allows for mixtures to go above RON 100. Ethanol, for example, has a RON of 109!
Your engine is designed to work with a minimum RON, which can be found by opening the fuel flap of your car. ‘Unleaded Petrol Only’ means anything above RON 91 petrol will work fine, while ‘Premium Unleaded Only’ requires gasoline of RON 95 or higher. Using RON 91 petrol in an engine designed for use with premium fuels is damaging in the long term. You can now impress your gas station attendant the next time you fill your tank!
U.S. Gas Station Ratings: Conversion Chart
In the U.S., unleaded gasoline typically has octane ratings of 87 (regular), 88-90 (midgrade), and 91-94 (premium) due to them using a petrol rating system that is the average of RON and MON (motor octane number, a different way to determine octane rating). A conversion is provided below:
| Type | Gasoline RON | U.S. Gas Station Ratings |
|---|---|---|
| 100% iso-octane | 100 | 100 |
| 100% ethanol | 109 | 99 |
| E85 gasoline | 102-105 | 94-96 |
| Premium or Super Unleaded (10% ethanol) | 97 | 92-93 |
| Premium Gasoline | 96-98 | 91-93 |
| Mid-Grade Gasoline | 94-95 | 89-90 |
| Regular Gasoline | 91-92 | 87 |

Tetraethyllead as an Antiknock Agent
In order to prolong their engine’s lifespan and reduce fuel consumption, people soon started looking for antiknock agents that could be mixed with gasoline to reduce engine knock. Tetraethyllead, discovered in the 1920s, proved to help fuel burn much cleaner, drastically reducing engine knock. It was promptly added to many gasoline mixtures.
However, the combustion of tetraethyllead produces lead, a highly toxic heavy metal. Lead is absorbed into the bloodstream and interferes with enzyme function. It can also cross the blood-brain barrier as lead is similar in size to calcium, a mineral our brain needs.
Tetraethyllead was quickly found to cause serious environmental and health effects, but the lead industry played them down. Fifty years would pass before these effects were finally exposed in the 1970s, with the world banning the use of tetraethyllead. However, its use continues even today, especially in some developing countries.
Ethanol in the Mix
Ethanol is a relatively cheap (and much less toxic) antiknock agent widely used today. RON 91 petrol containing 10% ethanol is known as E10, which improves the gasoline’s performance to around RON 94. Pure ethanol has an effective RON of 108, making it more efficient than 100% pure iso-octane.
However, ethanol releases about 40% less energy than petrol upon burning, which increases fuel consumption2. This means that running E10 means you will use 3% more fuel than if you used RON 91 petrol, but with better performance. Therefore if E10 at your gas station is more than 3% cheaper than RON 91, it’s a good idea to start using it! The same goes with E20 (if it’s more than 6% cheaper than RON 91). Most car engines will have no issues running with up to 20% ethanol in the gasoline mixture3.
Furthermore, carbon monoxide and unburned hydrocarbon emissions from gasoline combustion are massively reduced as we mix it with up to 20% ethanol. This reduction amounts to a 46.5% and 24.3% decrease in CO and HC emissions, respectively, across all engine speeds4. With such emissions driving climate change, perhaps we can do our part by filling our tank with E10 or E20 fuel the next time we’re at the gas station.

Reference
- Rudolph, A. M. (2003). Rudolph’s Pediatrics (pp. 904-906). Appleton & Lange.
- Wheals, A. E., Basso, L. C., Alves, D. M., & Amorim, H. V. (1999). Fuel ethanol after 25 years. Trends in biotechnology, 17(12), 482-487.
- Hilton, B., & Duddy, B. (2009). The effect of E20 ethanol fuel on vehicle emissions. Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automobile Engineering, 223(12), 1577-1586.
- Al-Hasan, M. (2003). Effect of ethanol?unleaded gasoline blends on engine performance and exhaust emission. Energy Conversion and Management, 44(9), 1547-1561.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




