Oral medication is often considered the ideal drug product as it is easy and convenient to administer. Extended-release tablets go one step further, extending the duration of the therapeutic effect. This article discusses how extended-release drug products work and their pros and cons.
Extended-release tablets slow down the rate of disintegration and dissolution of the medicine in the gastrointestinal tract. In doing so, they extend the time it takes for the active ingredient to be absorbed into the circulatory system.
Extended-Release Drugs Pros and Cons:
| Pros | Cons |
|---|---|
| Reduce overall doses (better patient compliance) | Higher risk of dosage issues (dose dumping) |
| Relatively easy to design and modify | Increased size of drug tablet/capsule |
| Expands market share for the parent company | ER forms are generally more expensive |
| Increase brand recognition and awareness | Insurers might not cover ER forms |
| Might be eligible for a market exclusivity period | Innovation issues in drug development |
What are Extended Release Drugs?
Oral Dosage Forms
When it comes to medication, oral drugs are by far the most common, representing 90% of the market share of pharmaceutical products worldwide and with a global market value of US$98.3 billion (2020).
This class of orally administered drugs can be divided into solid dosage forms (tablets, capsules) and liquid forms (syrups, tinctures, suspensions).
Solid dosage forms like tablets and capsules are considered the gold standard in drug delivery systems because they are easy to administer, manufacture, store and transport.
Pharmaceutical companies also prefer solid dosage forms to liquids because they are easier to design and allow for more extensive modification.
Standard vs. Extended Release vs. Delayed Release Drugs
Typical oral dosage forms contain the active ingredient and non-active components (excipients). They are designed to be swallowed by the patient and disintegrate in the stomach. The active ingredient dissolves in the gastrointestinal fluid and is absorbed into the bloodstream, where it can exert its therapeutic effect.
Standard-release drugs are the most common, where the active ingredient is released all at once, the absorption is immediate, and the maximum blood concentration is reached quickly.
In extended-release (ER) drugs, the active ingredient release is drawn out over a longer period. This allows for a slower buildup of drug concentration in the blood, which means the patient received a prolonged therapeutic effect (if the minimum effective concentration is reached).
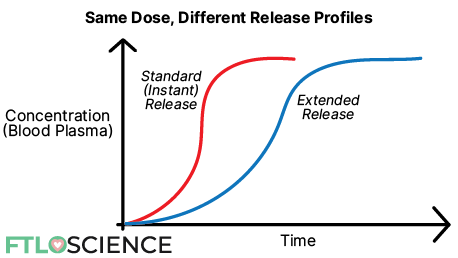
You can see from the graph above that although both the standard and ER forms have the same dose, the ER profile takes a longer time to reach maximum concentration.
Note: extended-release drugs are sometimes called prolonged-release or sustained-release drugs.
Closely related to extended-release forms are delayed-release drugs. These are designed only to release the active ingredient after a specific duration. Once this time has elapsed, drug release follows a typical profile.
Delayed-release forms are most often enteric-coated (gastro-coated) tablets, which have an outer layer resistant to the stomach’s acidic fluids. As a result, the active ingredient is only released when the drug reaches the higher pH (less acidic) environment of the small intestine, and the outer layer can dissolve.
How ER Oral Tablets Work
Conventional tablets break down quickly in the stomach, usually with the help of excipients known as disintegrants. Extended-release forms release the drug over 12 to 24 hours, which means a different approach to tablet formulation is needed. There are several ways to achieve this:
- Controlled diffusion. In this method, a membrane forms around the tablet after it comes into contact with water. This creates a diffusion layer with a concentration gradient. This membrane is usually a cellulose-derived polymer that is water soluble. The active ingredient has to pass from the bulk tablet into the gastrointestinal fluid through the diffusion layer, which controls the diffusion rate.
- Controlled dissolution. The drug ingredients must dissolve before they can be absorbed, so slowing down the dissolution also slows the uptake speed. This requires modifying the formulation to include water-insoluble excipients or altering the active ingredient into a poorly soluble salt.
- Matrix systems. We can also use a matrix polymer to physically control the tablet disintegration rate. The drug product is mixed with a polymer that degrades slowly in solution, freeing the active ingredient particles in the process.
Benefits of Extended-Release Drugs
Improves Patient Compliance and Convenience
ER drugs are designed to be beneficial to the patient. It can reduce the number of pills a patient takes while increasing the intervals between doses, which makes it particularly convenient for children (pediatric) or elderly (geriatric) patients.
In many cases, ER drugs have a higher dose of active ingredients than standard-release versions. This ensures the drug achieves the minimum effective concentration in the patient’s blood over the entire duration of action.
While this might sound unsafe, the formulation will have been tested extensively to ensure its safety and effectiveness. Before they can market different formulations of the original drug product, pharmaceutical companies must present clinical trial data specific to the new formulation.
Extends Therapeutic Benefits While Reducing Side Effects
Extended-release forms can maintain a therapeutic effect over an extended period, making them beneficial in situations where it would be inconvenient for the patient to take multiple doses.
One example would be pain relief medications, allowing patients to enjoy uninterrupted sleep after a single dose. ER drugs also allow for tailored drug release timings, such as taking the tablet at night to benefit from its effects in the morning (especially useful for arthritis, asthma, allergies, etc.).
Compared to standard-release drugs that are absorbed quickly, ER forms release their active ingredients slowly. This allows time for the body to remove the drug through metabolism while it is still being absorbed, leading to a lower maximum concentration.
For potent drugs with side effects at high concentrations, ER forms can reduce the likelihood and severity of these side effects.
Commercial Gain for the Parent Company
New formulations for existing medicines cost much less than developing a new drug from scratch. With every new drug product costing US$2 billion over 14 years of development, you can see why pharmaceutical companies want to extract as much revenue as they can from every approval.
Extended-release oral dosage forms are relatively easy to design and modify, which makes them attractive options. Despite their higher manufacturing cost, patients and insurance companies are willing to spend more on these ER forms compared to standard versions.
Pharmaceutical companies often run aggressive marketing campaigns for ER formulations, although this is often a point of contention. A study found that swapping 20 extended-release versions of drugs for their generic, immediate-release forms would have saved Medicare US$13.7 billion from 2012 to 2017.
Market Exclusivity for the Pharmaceutical Company
Another reason pharmaceutical companies produce extended-release versions of existing drugs is to take advantage of the market exclusivity period. When they develop a new drug, pharmaceutical companies are given a window of about ten years to sell their product exclusively. After this lapses, other companies can manufacture and sell their medicine as a ‘generic’ version—often much cheaper than the parent company.
To maximize their revenue, pharmaceutical companies design extended-release versions of a drug when it is nearing the end of this exclusivity period. They then file a New Clinical Investigation application with the FDA (for an existing entity with a different dosage regimen). If successful, the extended-release formulation can be granted up to 3 years of market exclusivity from the approval date.
One great example of this can be seen in Bristol Myers Squibb’s Glucophage (metformin) to treat type 2 diabetes. While the FDA approved the immediate-release form in 1994, Bristol Myers Squibb released an extended-release form, Glucophage XR, in 2000. This was followed by subsequent new formulations (see image below) of metformin in 2002, 2010 and 2014.

Drawbacks of Extended-Release Drugs
Dose Dumping
Although rare, one central area of concern in ER drug forms is the possibility of dose dumping: the premature release of the entire drug dose. Because ER drugs often contain a higher amount of active ingredients, this can lead to overdosing and adverse side effects.
Dose dumping occurs when the extended-release mechanism fails to deploy correctly, such as the premature disintegration of the matrix polymer. This can happen due to variations in the patient’s gastrointestinal environment (high/low pH), disease states, genetics and diet.
However, as mentioned above, pharmaceutical companies must perform safety studies to ensure the risk of dose dumping is minimal before they are allowed to market their ER product.
Variation in Bioavailability
Bioavailability is the proportion of active ingredient dose that makes it to the intended site of action. Extended-release drugs are designed to persist in the patient, which leads to a higher risk of changes in bioavailability due to interactions with food, secretions and other drugs in the gastrointestinal (GI) tract.
For example, eating food that is high in fat content can increase bioavailability by helping hydrophobic (non-polar) ingredients dissolve. However, fat molecules also delay stomach emptying, which slows down the drug’s absorption rate and can affect its concentration profile in the blood.
Because most ER drugs require water to function correctly, having sufficient fluid in the GI tract is critical. Disease states or other drugs can cause a reduction in stomach secretions and affect the ER drug release, hence causing variations in bioavailability.
ER Forms are Expensive to Produce
Cost is a significant factor in manufacturing extended-release tablets. Each unit costs 2 to 3 times more than a standard-release tablet, meaning patients must pay a high premium to switch from SR to ER forms. Pharmaceutical companies rarely invest in developing an ER version of a drug that isn’t doing well on the market.
Patients must balance their needs and the tangible improvement of switching with the extra cost. In many cases, the cost outweighs the benefits. This issue is compounded by insurers refusing to cover extended-release versions of a drug, especially when generic standard-release versions come at a fraction of the cost.
The number of modified-release oral dosage forms skyrocketed in 2005, as shown in the number of patents granted, but has not increased much since then, perhaps indicating a slowdown in this area.
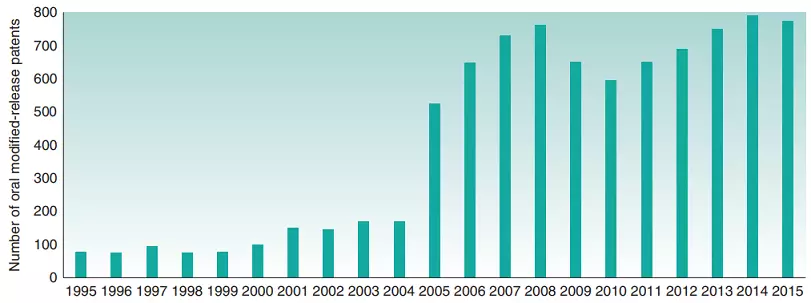
Despite this, the global extended-release drug market size is a whopping US$46 billion (2021), roughly half of the overall oral drug market. These high returns can lead to innovation issues if pharmaceutical companies focus on designing new formulations for existing drugs instead of developing novel therapies for other diseases.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.