Part of developing a drug involves choosing the proper packaging for it. Blister packs are convenient and keep pills free from contaminants. Plastic bottles are cheap but can react with the medication inside. Glass is expensive to produce and transport. With so many factors to consider, how do we decide what packaging to use?
Three main packaging types are used to store and transport drugs to the patient: blister packs, plastic bottles and glass bottles. The table below lists the main pros and cons of using each type of packaging. (Note: cost is estimated based on list prices of B2B suppliers; may vary)
| Blister Packs | Plastic Bottles | Glass Bottles | |
|---|---|---|---|
| Suitable Dosage Forms | Pills and capsules | Pills and capsules, liquid dosage forms | Pills and capsules, liquid dosage forms, sterile injections and infusions |
| Cost Per Unit Dose | 1 cent | 0.2 cents | Type 1: 10 cents Type 2: 3 cents Type 3: 0.4 cents |
| Weight Per Unit Dose | 0.3 g | 0.28 g | 20 g (single-use vial) 1.25 g (multi-use) |
| Contamination Risk | Low | High | Lowest (single-use) Medium (multi-use) |
| Reactivity | Medium | High | Lowest (type 1) Low (type 2 and 3) |
Types of Drug Packaging
Blister Packs
The most common way to supply oral pills to patients is through blister packaging. Blister packs have plastic grooves with an aluminium backing, usually holding between 1 to 30 pills. Each tablet is housed so that its aluminium backing is punctured before administration of the drug.
Blister packs are convenient for the patient and simple to use while being easy to store and transport. They also reduce the chance of contamination since only one pill is exposed at a time. We can also print instructions and designs onto blister packaging, such as in 28-day birth control pills.
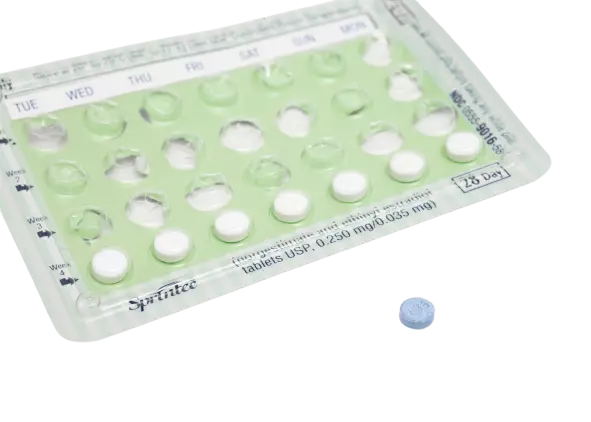
We can improve the recyclability of blister packs by using plastics like polyethylene (opaque forms also prevent light damage). Using thicker sheets of plastic and aluminium can better protect the pill during storage and transport, but these modifications come at a cost.
Plastic Bottles
While blister packs are single-use containers, bottles are usually multi-dose—containing several pills inside—which makes them more cost-effective. Plastic is also lightweight and shatterproof, which benefits transport and shipping.
Bottles also have the advantage of being suitable for liquid dosage forms. However, plastic is permeable to gases and some liquids. Plastic might not be compatible with some drugs, as it is reactive to solvents and acids/bases, which might contaminate the drug or reduce its effectiveness.
There are a range of different plastics polymers used in pharmaceutical packaging, each with different properties. They can be produced either as thermoplastics (polymer chains) or thermoset polymers (cross-linked polymers).
Thermoplastics can be remolded into different shapes due to their chemical structure, while thermoset polymers have a fixed shape. Both categories of plastics have different uses in pharmaceutical manufacturing.
Type 1 Borosilicate Glass
Glass bottles chemically inert compared to plastic bottles and are impervious to liquids and gases in the environment. Glass bottles can also be coated with different colors, protecting their contents from light damage. This makes glass an ideal material for drug packaging.
On the other hand, glass is the most expensive material to produce as well as the heaviest. It is also more fragile than plastic containers and blister packs, which makes handling and storage slower and costlier.
Glass bottles come in different grades, with borosilicate glass being the most stable (and expensive). It is made by introducing boric oxide into the silicon dioxide structure during manufacturing, forming a stable structure that resists chemical reactivity and thermal expansion.
Borosilicate glass in pharmaceutical manufacturing is known as type 1 glass, the highest grade available. It is almost exclusively used to store reactive liquids or sterile preparations and rarely for solid dosage forms.
Type 2 Glass
Soda-lime glass is a cheaper alternative to borosilicate (type 1) glass but is more reactive due to sodium and calcium oxides (alkaline chemicals) in the structure.
We can treat soda-lime glass with sulfur dioxide that reacts with these alkaline compounds, converting them into sulfate salts. The salts are then washed away during the manufacturing process. This prevents the alkaline chemicals from reacting with drug preparations, also known as leeching.
Treated or dealkalized soda-lime glass is known as type 2 glass, the next grade of pharmaceutical glass. It is used as drug packaging for non-acidic liquid drug forms (acid can still react with the alkaline oxides over time).

Type 3 Glass
Untreated soda-lime glass (type 3 glass) is the cheapest to produce; most household glassware is made with this glass.
Soda-lime glass is unsuitable for many preparations as the surface chemicals can leech into solutions, contaminating them. Pharmaceutical regulations ban type 3 glass for intravenous drugs, where maintaining the sterility of the drug product is essential.
Type 3 glass still finds widespread use in non-sterile liquid drug forms like syrups and tinctures. Some powders are sold in type 3 glass bottles, where patients reconstitute them with water right before taking them.
Comparing Between Drug Packaging Types
Cost
Developing a new drug costs roughly US$2 billion. With this in mind, pharmaceutical companies must reduce costs as much as possible to make medicine affordable.
- Blister packs can cost as little as 1 cent per pill (based on 10 cents for a 10-pill PVC blister pack with aluminium backing).
- Plastic bottles for medicine cost around 20 cents each, which equates to 0.2 cents per pill (based on 100 tablets in 200 ml bottle), by far the cheapest packaging option for drugs.
- Type 1 and 2 glass are primarily used for single-use sterile preparations and cost 10 cents and 3 cents, respectively, making them the most expensive packaging per dose.
- Type 3 untreated soda-lime glass is used as a multi-use pill or capsule container that costs 40 cents, or 0.4 cents per dose (based on 100 tablets in a 200 ml bottle).
Weight
- Blister packs weigh 3 g for the 10-pill plastic container and aluminium backing, or 0.3 g per dose. The exact dimensions will depend on the shape and size of the tablets.
- Plastic bottles are the lightest packaging available. A 200 ml HDPE bottle with PP child-resistant cap weighs 28 g, or 0.28 g per dose (based on 100 tablets in the 200 ml bottle).
- Single-use type 1 and glass vials weigh 20 g each, making them the heaviest packaging per unit dose. This means they are also the most expensive to transport.
- Type 3 glass containers for multi-use weigh around 125 g for a 200 ml bottle, which equates to 1.25 g per dose.
Contamination Risk
Blister packs are great because only one pill is exposed at a time, with the others in the package remaining sealed, protecting against air/water damage and microbial contamination.
In multi-use bottles, contamination is a more significant risk because each dose the patient takes exposes the rest of the drug contents to the environment.
We often see desiccant sachets inside multi-use bottles to draw water away from the pills, which increases their shelf-life. Storing multi-use bottles in the fridge also helps slow the growth of bacteria and fungi.
Single-use, low-reactivity glass bottles (types 1 and 2) carry the lowest risk of contamination. Glass can also be sterilized under high heat (unlike plastics), which is why they are used for injections and infusions where the drug directly enters the patient’s bloodstream.
Reactivity
The issue with blister packs is the reactivity of the aluminium backing. Although cheap to produce, aluminium is a relatively reactive metal, which can contaminate or decrease the shelf life of the drug product. We can apply a resin coating to decrease the reactivity of the metal backing.

Plastic bottles are unsuitable for many applications because they absorb and adsorb chemicals. For example, many drug formulations contain anti-oxidants as preservatives. If the plastic material absorbs the anti-oxidant, it will reduce the drug’s stability and shelf life.
Plastic bottles may also be reactive, though this depends on the type of plastic used:
- Polyethylene (PE): most common plastic but permeable to oxygen, not suitable for oxygen-sensitive drug formulations. Low-density PE is usually used in blister pack containers (flexible); high-density PE in rigid bottles.
- Polypropylene (PP) is less reactive than PE and is moisture-resistant, which makes it suitable for storing liquid drug preparations.
- Polystyrene (PS) is less common in pharmaceutical packaging since moisture and gas can penetrate its surface. It can still be used for tablets and capsules that aren’t sensitive to either.
Borosilicate glass is unreactive—that’s why most laboratory reagents come in borosilicate glass bottles. However, type 2 and 3 soda-lime glass contain surface alkaline chemicals that can react with pills or liquids. Type 2 glass is less reactive than type 3 but should not be used to store acidic solutions.
Tamper-Proofing
We often take for granted that our medicines come as intended by the manufacturer. Through negligence or malicious intent, harmful chemicals can enter containers and contaminate the drugs. There are several ways we can design drug packages to be tamper-proof.
Bottles can be tamper-proofed using special seals with a warning to discard the contents if the seal is damaged. Tamper-proof seals help ensure the product’s safety within the plastic or glass bottle.
Blister packs are tamper-proof since any damage to the material is quickly apparent. We should discard the pill if we find any damage to its plastic cover or aluminium backing! The same applies to single-use bottles that contain sterile liquids for injection.
Child-Resistant Packaging
Regulatory requirements require most drug containers to have child-proof mechanisms. In bottles, this is usually in the form of a childproof cap that must be pushed down before turning, preventing children from opening them.

Blister packs don’t have child-resistant features, which makes them less safe. Tablets and pills are also often manufactured with colorful designs, causing children to mistake them for candy.
While some ingenious child-resistant blister packs are available for more potent drugs, children who come across blister packs are unlikely to swallow multiple pills quickly (compared to drinking liquid dose formulations in bottles).
Which Drug Packaging to Choose?
Multi-use plastic bottles are the most cost-effective option for pharmaceutical containers, being cheap to produce and weighing the least per unit dose. However, the chemical compatibility of the active ingredient and any excipients used to manufacture the tablet or formulation must be studied.
Blister packs remain the most common packaging method for tablets and capsules as they are single-use (reducing contamination) while being lighter and cheaper than glass bottles.
Type 1 and 2 glass bottles are expensive, so pharmaceutical companies only use these for sterile liquids. Type 3 glass is used for pills and tablets sensitive to light, air or moisture.
We can identify suitable drug packaging early in the drug development process, modifying the drug’s chemical composition to save packaging costs, translating to cheaper drugs for the patient.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.