Drug polymorphism occurs when a single chemical compound arranges itself into multiple crystal polymorphs under different conditions. It is a required field of study within pharmacology, as polymorphic forms can drastically change a drug’s safety and efficacy. This article discusses drug polymorphs and how they can be controlled in pharmaceutical manufacturing.
What is Polymorphism?
Same Chemical, Different Structures and Properties
Structural polymorphs are identical molecules that can form different crystal arrangements. Depending on how a chemical is packed in a bulk solid, it can form various crystal structures (polymorphs) with different structures and properties.
Molecules must form repeating arrangements known as unit cells before they can be considered polymorphs. Solids that do not have a defined unit cell are known as amorphous (non-crystalline). For example, silicon dioxide (SiO2) in crystal form is known as sand, but in its amorphous form is known as glass.

When it comes to drug molecules, these polymorphs can have a significant effect on the patient. Different polymorphs can have vastly different bioavailabilities (the fraction of the total drug that reaches the therapeutic site) and even toxicity profiles.
Polymorphism is also a crucial aspect from a manufacturing standpoint, as different forms can influence the stability and physical properties of the drug. It is therefore essential to understand and control the formation of drug polymorphs.
Types of Polymorphism
There are 3 ways to classify polymorphs: Packing, Conformational and Pseudopolymorphism.
Packing Polymorphism
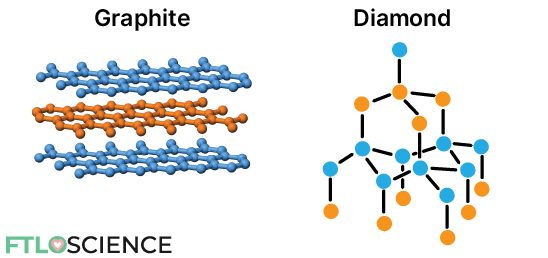
Rigid, inflexible molecules form polymorphs by arranging themselves differently, also known as packing polymorphism. Graphite and diamond are polymorphs consisting of rigid carbon lattices packed differently. As a result, the graphite form of carbon is much ‘softer’ and a better conductor of electricity than the diamond form.
The analgesic drug paracetamol is another example of packing polymorphism, with at least 3 distinct polymorphs.
Conformational Polymorphism
Conformational polymorphism becomes possible when molecules have long hydrocarbon chains or other flexible groups. These groups allow the molecule to adopt different shapes, forming conformational polymorphs.
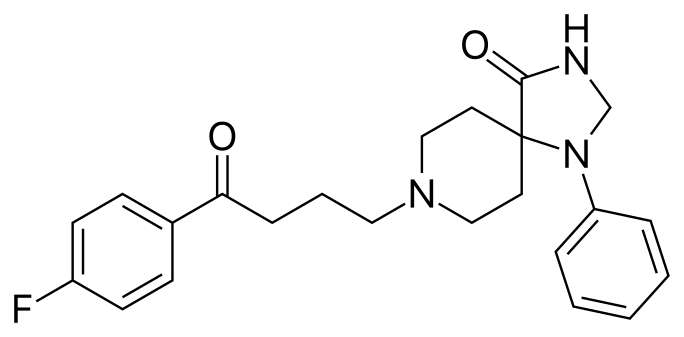
The antipsychotic drug Spiperone has a long aliphatic (single-bond carbon) chain that can either be linear (form 1) or form a 90° angle (form 2).
Hydrates and Solvates (Pseudopolymorphs)
Solvent molecules often incorporate themselves into polymorphs, forming solvates or hydrates (if the solvent is water). These solvates are considered pseudopolymorphs, as they cannot form without the help of solvent molecules to stabilize their structure.
When molecules can form both, pseudopolymorphs are generally less soluble (but more stable) than their crystal polymorph counterparts.
How do Polymorphs Form?
There are several mechanisms through which crystal polymorphs can form:
- Spontaneous crystallization (nucleation): when a pure substance is dissolved in a solvent, the molecules can arrange themselves spontaneously in an ordered fashion. This process can be accelerated by lowing the temperature of the solution. Many ‘crystal growing’ experiments form through spontaneous nucleation.
- Recrystallization: polymorphs that are dissolved and allowed to recrystallize can often result in a different form, depending on the solvent and other physical parameters.
- Transformation: polymorphs can ‘morph’ into different forms, depending on temperature, pressure, humidity, concentration, and other external factors. Over time, molecules that can exist in multiple polymorphs will transform into the most stable polymorphic form.
- Solid-state reactions: in the absence of solvents, molecules that come together can react in solid-state reactions. The product is a new compound that might have a different structure. In drug tablet manufacturing, the dry ingredients can react due to compression, forming new molecules.
Although some molecules can form a multitude of polymorphs, many of them are unstable, with the molecules rearranging themselves into more stable polymorphs. Over time, all polymorphic forms of a molecule will converge into a single, most thermodynamically stable form.
Pharmaceutical companies must characterize and study possible drug polymorphs during the drug development stage to better understand their safety and efficacy. Regulatory agencies like the FDA often view such studies favorably when reviewing drug applications. In some cases, polymorphic forms of a drug can influence the scope of intellectual property and patent protection.
Polymorphs in Medicine
Physiological Effects of Drug Polymorphism: A Case Study
Polymorphism in drug products is a critical area of research since drugs switching between different polymorphs can alter their intended effects on the patient. Mebendazole, a drug for treating parasitic infections, exists in three conformational polymorphic forms, shown below.

A 2020 study of Mebendazole’s polymorphs showed that the log P of form B was much lower (2.25) than forms A and C (2.40), which makes it more soluble in water. This leads to form B being highly toxic in patients. An electron density plot shows that form B has areas of higher electronegativity compared to forms A and C.

The values suggests that forms A and C have similar stabilities and solubilities. However, they have different pharmaceutical properties. While form C is the therapeutically effective form, form A has poor patient bioavailability with little therapeutic effect.
The case study of Mebendazole supports the claim that while solubility and stability play a big role in influencing therapeutic outcomes, polymorphic forms alone do not tell the whole story.
Quality Control and Stability Testing
It, therefore, becomes important to control polymorphs to ensure the quality and safety of drug products. The different physical properties of polymorphic forms allow us to develop methods to probe their identity; these include:
- Moisture content analysis
- Solubility studies
- Solid-state chemistry
- Crystallography techniques
- Particle size analysis
Because polymorphs transform over time to the most stable form (which might not necessarily be the ideal therapeutic form), it is also critical to test them in stability studies. This ensures that patients receive therapeutic benefits from the drug product, and not an ineffective polymorph or worse, a toxic dose.
If a drug product transforms from their therapeutic polymorph too quickly, there are several ways we can slow down the transformation:
- Altering the active pharmaceutical ingredient (API)
- Modifying the formulation (replacing or removing excipients)
- Changing the manufacturing processes (milling, grinding, compression, coating, etc.) to reduce temperature and pressure changes
- Improving storage conditions (preventing moisture/oxygen/light damage)
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.