The earliest record of glass production dates to 3600 BC in the Mediterranean, later finding widespread use through the Roman Empire’s large-scale manufacture of the material. Its stability and chemical characteristics make it an excellent material with many applications. We can also incorporate specific chemicals into its amorphous structure, producing colored glass.
Glass is usually transparent, but we can insert metals and metal ions into its structure to create colors. We can use several molecules to give glass color; they are often mixed at different concentrations to achieve different hues. The most common additives are shown in the table below, along with their corresponding color.
| Metal or Metal Ion Additive | Color Produced |
|---|---|
| Antimony | Yellow to red |
| Cadmium sulfide | Deep yellow |
| Chromium | Green to black |
| Cobalt oxide | Deep blue |
| Copper | Turquoise |
| Copper oxide | Dark red |
| Didymium | Green to red |
| Gold | Red |
| Iron oxides | Blue-green |
| Lead | Purple |
| Manganese | Amethyst purple |
| Manganese dioxide | Black |
| Nickel | Blue, violet or black |
| Tin | White |
| Titanium | Color enhancer/brightener |
| Uranium oxides | Yellow-green |
The Chemical Structure of Glass
Glass isn’t a Crystal
Despite being transparent and shiny, glass isn’t a crystal! Not in the chemical sense, anyway. Crystalline compounds in chemistry have an ordered structure with clearly defined repeating units. Sodium chloride (table salt) is a crystal network of sodium and chloride ions.
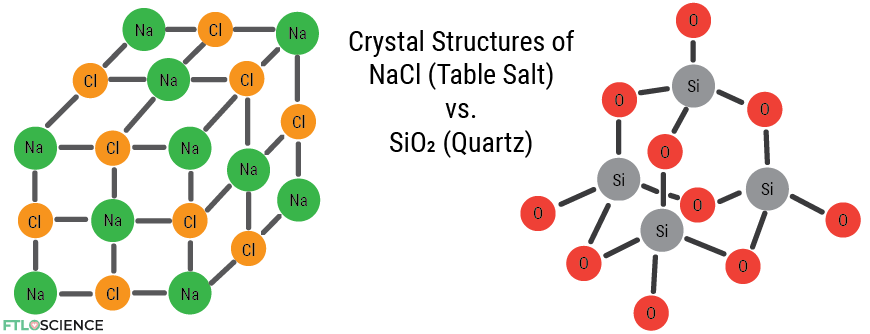
Glass is made by melting sand, which consists primarily of quartz crystals. Quartz is a network of silicon dioxide (also known as silica) molecules.
At high enough temperatures (1700 °C), the ordered network of silicon and oxygen atoms falls apart, forming glass. Glass is an amorphous structure, which means its atoms are in a non-crystalline, random arrangement.
Why is Sand Opaque but Glass Transparent?
You might wonder why sand (a crystal) is opaque while glass (a non-crystal) is transparent! This is because, in a crystalline structure, silicon dioxide has electrons that can absorb light of a particular wavelength that we see as color.
When this crystal structure breaks down, the electrons absorb photons at a lower wavelength (higher energy) that no longer corresponds to visible light. This means that colored light passes through glass, making it appear transparent. This is also the reason why many organic compounds appear white or transparent.

Chemical Additives in Glass
The absurdly high temperature needed to melt pure sand into class makes the process unfeasible. The Ancient Romans discovered that adding sodium carbonate (also known as soda ash) to the mix lowers this temperature to around 1000 °C.
Potassium carbonate (potash) works similarly, sitting within the silica network and forcing the atoms apart, preventing the crystal structure from reforming. Chemicals that can lower the melting temperature of glass are known as fluxing agents.
We can also incorporate other chemicals into the glass mixture to change the properties of the final product. For example, calcium oxides from limestone stabilize the glass and prevent water damage.
The glassware we most commonly encounter today contains 70% silicon dioxide (silica), 15% sodium oxides (soda) and 9% calcium oxides (lime). This is where it gets its name, soda-lime-silica glass—or simply soda-lime glass.
Borosilicate glass, like pharmaceutical grade type 1 glass and laboratory glassware, is chemically inert and resistant to thermal expansion, thanks to the addition of boric oxides to its structure.
Chemical Additives Make Glass Colorful
How Do We Produce Color in Glass?
Apart from durability and stability, we can also alter the color of glass by adding certain chemicals to the mixture. Colored glass is useful in various settings, from stained glass windows and decorative glassware to storing chemicals that need protection from light damage.
Despite the ‘stained’ glass nomenclature, no actual staining occurs when we produce colored glass. If we simply painted on the surface of the glass, it would lose its transparency!
Instead, we can add metal and metal ions into the glass structure as part of the melting mix during manufacture. We only need a small amount (less than 1% of the total mixture) to create colored glass, as long as they are thoroughly mixed.
The metals that produce color are usually elements found in the periodic table’s d-block (transition metals) and f-block (lanthanides and actinides). These elements have a larger atomic radius, hence require less energy to excite their electrons. This means they absorb and emit photons corresponding to visible light, producing color.
List of Metals Used for Coloring Glass
Below is a table of metals and metal ions (usually in the form of their oxides) commonly used to produce color in glassware. The shade of color can change with concentration. As we will discuss later, we often find a mixture of additives in colored glass that provide a specific hue.
| Metal or Metal Ion Additive | Color Produced |
|---|---|
| Antimony | Yellow to red |
| Cadmium sulfide | Deep yellow |
| Chromium | Green to black |
| Cobalt oxide | Deep blue |
| Copper | Turquoise |
| Copper oxide | Dark red |
| Didymium | Green to red |
| Gold | Red |
| Iron oxides | Blue-green |
| Lead | Purple |
| Manganese | Amethyst purple |
| Manganese dioxide | Black |
| Nickel | Blue, violet or black |
| Tin | White |
| Titanium | Color enhancer/brightener |
| Uranium oxides | Yellow-green |
Applications of Colored Glass
The First Colored Glass
The first known additive used to color glass was manganese, which lent a lovely amethyst purple hue to the Ancient Egyptian glassware. Today, low concentrations of manganese are added to glass contaminated with iron, removing its green tint.
Manganese is versatile because it has a range of oxidation states, producing transitions corresponding to various colors. For example, manganese dioxide (+4 oxidation state) has been used to color glass black for hundreds of years.
However, manganese slowly reacts with sunlight and oxygen in the environment to produce sodium permanganate—manganese in a +7 oxidation state—that is purple. As a result, old windows that contain manganese eventually turn into beautiful violet-colored stained glass panels.
Beer and Wine Bottles
Alcoholic drinks often need to be protected from light. Hop-derived compounds in beer and fermentation products in wine are often sensitive to light, forming new compounds due to light exposure. These compounds are generally unpleasant and associated with skunked beer and wine faults.
Iron (II) oxide is often added to beer bottle glass, which gives it a bluish-green tint. In wine bottles, chromium can be added with iron oxide to give a darker green hue, as wine compounds tend to be more sensitive to light.

Laboratory Glassware and Pharmaceutical Packaging
Many chemical reagents and medicines are sensitive to air, water and light damage. Hence, borosilicate glass is considered the gold standard packaging material for drugs as opposed to plastics that are more permeable to air and water.
However, the borosilicate glass used to store such chemicals must protect its contents from light damage. Mixtures of metal oxides are added to make tinted amber and brown borosilicate glass bottles.
Amber glass bottles use a mixture of iron, manganese and carbon oxides with sulfur compounds to provide the tint. The brown-colored bottles are made similarly but without manganese, making them less effective at blocking light. We can create an effective black tint by combining iron, manganese and cobalt during glass manufacture.
In general, increasing the number and concentration of additives will darken the overall color of the final product. Using several different metals and metal ions ensures that a broader range of visible light is absorbed, making the glass appear as dark as possible.

Eye Protection and Photochromic Glass
We can also design mixtures that allow the glass to block specific colors, such as the yellow light (589 nm wavelength) that heated sodium emits. The additive, in this case, is didymium—a combination of the lanthanide elements praseodymium and neodymium.
Safety glasses made with didymium glass protect the wearer’s eyes from being blinded by the intense yellow flashes of light emitted from forges commonly used in glassblowing and blacksmithing. Because the other colors pass through the glass, the wearer maintains good color vision, unlike with general dark-tinted glasses.
Photochromic lenses (also known as transition lenses) use silver ions to achieve a darkening effect when exposed to sunlight. In the presence of ultraviolet light, the transparent silver ions in the glass react to form opaque silver metal atoms. When photochromic glass is in the shade, the silver metal reforms silver ions, and the glass appears colorless.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.