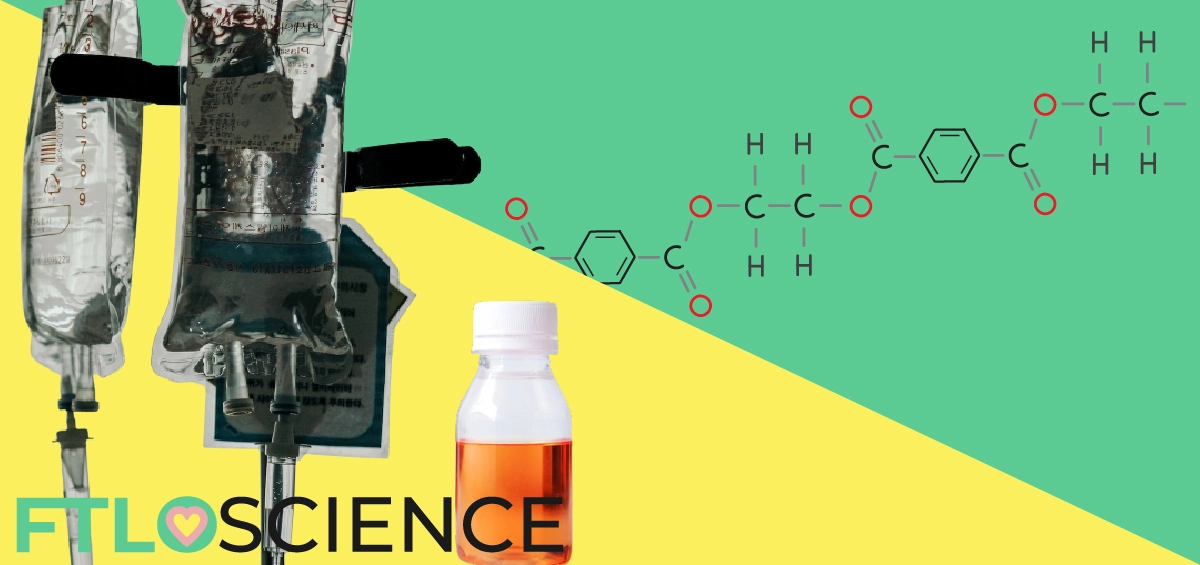
The discovery of plastic over 100 years ago was a manufacturing miracle. Being cheap to produce yet versatile, plastic is still the most popular material in almost every industry; pharmaceutical packaging is no exception. Both solid and liquid medications often come in plastic bottles, but how do we decide what type of plastic to use for each drug product?
There are eight main types of plastics we can use to package pharmaceutical drug products, each with unique properties and applications. They are listed in the table below; you can click on the links to jump to the section discussing each plastic type. A more detailed comparison table is provided at the end of the article.
| Plastic Type | Chemical Structure | Suitable For |
|---|---|---|
| High-Density Polyethylene (HDPE) | 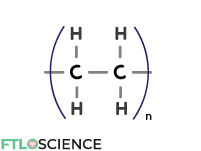 | Solid dosage forms |
| Low-Density Polyethylene (LDPE) |  | Blister packs |
| Polypropylene (PP) | 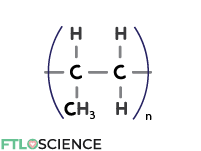 | Wide range of applications |
| Polystyrene (PS) | 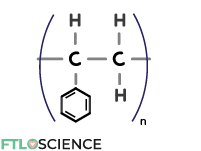 | Limited |
| Polyethylene terephthalate (PET) | 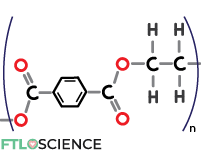 | Liquid dosage forms |
| Polyvinyl chloride (PVC) | 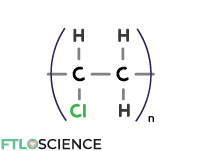 | Sterile drug products |
| Polyvinylidene chloride copolymers (PVDC) | 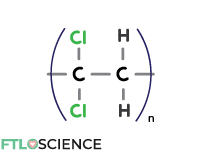 | Barrier material, used as coating |
| Polychlorotrifluoroethylene (PCTFE) | 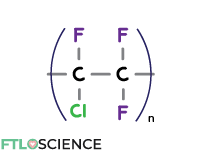 | Barrier material, used as coating |
How Plastic is Used in Pharmaceutical Packaging
Plastic Bottles and Blister Packs
When it comes to pharmaceutical packaging, we have several options available. Blister packs provide a convenient way to store and transport solid drug products like tablets and capsules. For liquid dosage forms like cough syrups, however, we must use either plastic or glass bottles.
Glass bottles are inert, so they are unlikely to react with the drug product stored within. Glass can also be colored to protect its contents from light damage. However, glass as a material is heavy and expensive to produce, so manufacturers often prefer using plastic where possible.
If you are interested in the topic of drug packaging, we have resources to help you choose between different drug packaging types and understand tamper-proofing regulations.
Plastic bottles offer the cheapest and lightest form of pharmaceutical packaging, so it is the obvious material of choice. However, plastic as a material can be reactive and permeable to gases and liquids, limiting its suitability for storing many drugs.
Plastics are Polymers
To begin, let’s look at the chemistry of plastic. Plastic is a polymer, which means it consists of long strands of repeating units—or monomers. If all monomers are identical, it is known as a homopolymer. If the mixture has different monomers, the result is a copolymer.
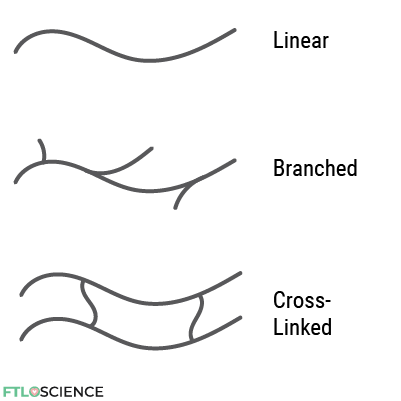
We can arrange monomers into long, unbranched strands (linear polymers) or create certain degrees of branching (branched polymers). Cross-linking between polymer chains is also possible. A cross-linked polymer behaves as a single molecule and can have a molecular mass of over 1,000,000 grams per mole!
Thermoplastic vs. Thermosetting Plastic
Linear and branched polymers have thermoplastic properties, which means they can be melted and reformed multiple times without deforming. Cross-linked polymers, on the other hand, are usually thermosetting plastics, which harden irreversibly once it is set into a mold.
The reason for this is the different types of bonding between polymers. In linear and branched polymers, weak intermolecular forces like hydrogen bonding and dipole interactions hold them together.
Because these bonds are weak, we can break them with low heat, converting the plastic into liquid form. While in liquid form, we can remold the thermoplastics into different shapes that harden when cooled.
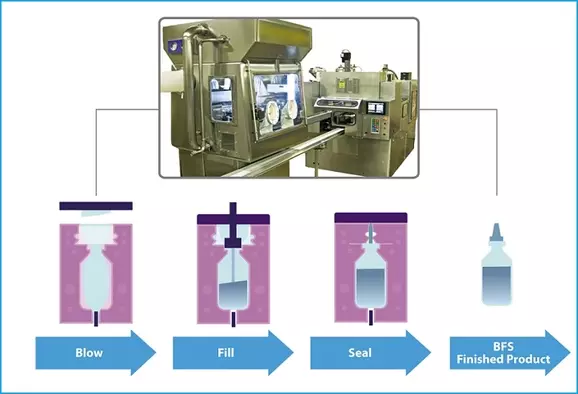
Thermoplastics are ideal for containing liquid dosage forms in pharmaceutical manufacturing, where the melting, cooling, filling and sealing steps can occur in a few short seconds using blow-fill-seal (BFS) technology.
Cross-linked polymers contain a network of covalent bonding between polymer strands; breaking these bonds requires much higher temperatures. Furthermore, we cannot reform the covalent linkages between the polymer strands once broken, which means thermosetting plastics become permanently deformed when heated to the melting point.
However, the one upside to thermosetting plastics is that they tend to be a more robust material than thermoplastics. Covalent cross-linking between the polymers gives the material a higher melting point, better chemical resistance and mechanical strength, making thermosetting plastics useful as adhesives and protective coatings.
Plastics Types For Pharmaceutical Packaging
Polyethylene (HDPE/LDPE)
By far the most common plastic (34% of the total plastics market), polyethylene (PE) comprises repeating units of ethylene (C2H4) linked through an addition polymerization reaction.
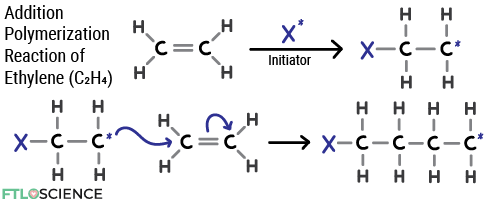
Linear PE polymer strands pack tightly together, forming high-density polyethylene (HDPE). HDPE is the most common plastic for solid pharmaceutical products, creating moisture-resistant and structurally rigid containers.
Branched PE polymer strands don’t pack as tightly; ethylene units branching away from the primary chains keep them apart. The result is low-density polyethylene (LDPE), a flexible and transparent material used in blister packs.
However, there are issues with using PE for pharmaceutical packaging. The material is permeable to air, making it unsuitable for storing oxygen-sensitive products. The ethylene units are also reactive to hydrophobic molecules and solvents, which can reduce the shelf-life and effectiveness of the drug product.
Polypropylene (PP)
We can replace a single hydrogen atom on the ethylene monomer with a methyl group (CH3) to form polypropylene (PP).
The extra methyl group makes PP more resistant to moisture and gases than PE, which means it is a suitable material for storing liquid drug forms.
The shape of the polymers increases the interactions between polymers, giving PP better stability. PP plastics can be autoclaved, extending their applications to include sterile drug products.
Polystyrene (PS)
Replacing the hydrogen in PE with a benzene ring produces polystyrene (PS). PS can take on many forms, from the opaque PS foam used in food packaging to transparent crystal PS used in disposable cups and plastic jars.
PS finds limited use in supplement containers or foam pellets for impact absorption during transport. Because of its poor chemical resistance and permeability to gases and liquids, it is rarely used as primary pharmaceutical packaging to store drug products.
Polyethylene Terephthalate (PET)
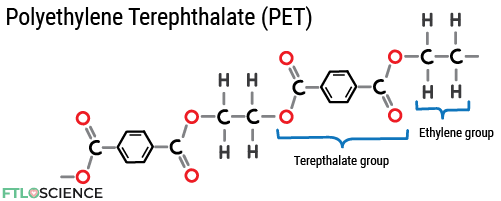
Sticking a terephthalate group into the polyethylene structure forms polyethylene terephthalate (PET). PET is more expensive to produce than PE, but it provides a transparent and rigid material for bottles. PET plastics can also withstand high temperatures while being a good gas and moisture barrier.
The ubiquitous plastic bottles used to store carbonated drinks are made with PET plastic as it prevents the dissolved carbon dioxide from escaping. In pharmaceutical manufacturing, PET containers commonly hold oral liquid dosage forms (syrups, emulsions, suspensions).
Polyvinyl Chloride (PVC)
To get around the reactivity issue around ethylene monomeric units, we can replace a hydrogen atom with chlorine, forming polyvinyl chloride (PVC). This makes PVC less reactive to non-polar liquids compared to PE and PP.
Because it is a transparent and flexible material, PVC is ideal for storing intravenous liquids like glucose and saline solutions. It can also be used as the plastic covering in blister packs.
Polyvinylidene Chloride Copolymers (PVDC)
We can replace a second hydrogen atom in polyethylene with another chlorine atom, forming polyvinylidene chloride copolymers (PVDC). The second chlorine atom in the repeating unit produces a polymer that is an even more effective moisture and gas barrier.
Like PVC, PVDC produces a transparent, flexible packaging material with good solvent resistance. Cling wrap was once made with PVDC but has been replaced with LDPE due to environmental concerns.
Polychlorotrifluoroethylene (PCTFE)
Going one step further, we can replace the rest of the hydrogen atoms on the monomeric units with fluorine, a very stable functional group, forming polychlorotrifluoroethylene (PCTFE). Substitutions with other halogens (chlorine, fluorine, etc.) are also possible, forming polymers with similar properties.
PCTFE has everything we want in pharmaceutical packaging; a transparent, gas/moisture-resistant material that can withstand high temperatures and organic solvents. It is also the most expensive type of polymer for this purpose.
Comparing Properties and Costs of Plastic Types
Below is a comparison table showing all eight plastic monomer types used below. Costs have been estimated from a wholesale supplier; actual prices may vary based on polymer structure and purity.
| Chemical Structure | Air Protection | Moisture Protection | Heat Tolerance | Transparency | Cost Per Ton | Suitable For | |
|---|---|---|---|---|---|---|---|
| High-Density Polyethylene (HDPE) |  | Poor | Good | High | Translucent to opaque | US$1000 | Solid dosage forms |
| Low-Density Polyethylene (LDPE) |  | Poor | Poor | High | Clear | US$1200 | Blister packs |
| Polypropylene (PP) |  | Good | Excellent | High | Clear | US$1200 | Wide range of applications |
| Polystyrene (PS) |  | Poor | Poor | Low | Clear to opaque | US$1400 | Limited |
| Polyethylene terephthalate (PET) |  | Good | Good | High | Clear | US$2500 | Liquid dosage forms |
| Polyvinyl chloride (PVC) |  | Good | Good | Low | Clear | US$1200 | Sterile drug products |
| Polyvinylidene chloride copolymers (PVDC) |  | Excellent | Excellent | High | Clear | US$1800 | Barrier material, used as coating |
| Polychlorotrifluoroethylene (PCTFE) |  | Excellent | Excellent | High | Clear | US$3000 | Barrier material, used as coating |
Plastics offer a versatile and cost-effective option for pharmaceutical packaging, with different monomers available to suit a range of drug types. Although not as chemically inert as glass, plastics provide a cheaper containment option that is easier to manufacture and transport.
However, it is vital to perform stability studies to evaluate materials for their compatible with each drug component (for example, organic dyes used in tablet manufacturing). Suitable plastic packaging should:
- Have limited reactivity with the active ingredient and excipients
- Not leech chemicals into the drug product (predominantly liquid forms)
- Not build up electrostatic charge during storage and transport
- Be compatible with the solvent used, pH, storage environment (humidity/temperature)
- Provide an appropriate shelf life to the drug product—the time it takes for a 10% drop in active ingredient concentration—that is generally between 1 to 5 years
- Be recyclable and have limited environmental impact
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.