Tablets are the most common form of drug delivery; many of us take pills regularly, whether as drugs or supplements. Pharmaceutical companies also favor tablet manufacturing because the process is usually cheaper and faster compared to producing other drug forms. This article will explore how pills are made and the ingredients that go into making them.
Tablets, or pills, are solid medications made by compressing powders. The drug in its powdered form is mixed thoroughly and filled into tiny molds, where compression machines use high pressures to fuse the powder into compact tablets. Tablet manufacturing machines can churn out hundreds of thousands of pills every hour, making it a cost-effective way to produce medicine.
The Tablet Manufacturing Process
Mixing the Ingredients
Tablets consist of the active pharmaceutical ingredient (API or just ‘active ingredient’) mixed with other substances, known as excipients. The active ingredient provides therapeutic benefits to the patient, while the excipients are inert, supporting the drug’s release in our body. Several examples of excipients and their functions are outlined in the table below:
| Excipient | Function | Examples |
|---|---|---|
| Filler | Forms the bulk of the tablet, ideally cheap and unreactive | Lactose, sugars, dicalcium phosphate, processed cellulose |
| Binder | Holds tablet together after compression | Starch, processed cellulose, gelatin, polymers |
| Lubricant | Helps with powder flow properties, prevents caking | Magnesium stearate, talc, sodium lauryl sulfate |
| Disintegrant | Aids disintegration of the tablet in the body (upon contact with water) | Sodium bicarbonate, povidone, cellulose polymers, starch |
| Coloring Agent | Provides a unique feature to an otherwise white tablet | Various organic dyes and colorings |
Once the ingredients are measured, they must be even mixed uniformly so that there is an exact amount of each component in the tablet. Since tablets are so small, any irregular mixture distribution greatly increases the likelihood of producing ones with too much of the drug (overdosing) or too little (underdosing).
Particle size is crucial in ensuring even mixing; if there is a big difference in particle sizes, the larger particles can sink to the bottom of the mixture (known as demixing). This causes uneven distribution of the active ingredient in the mix, potentially manufacturing tablets of different strengths.
Before moving on to the direct compression stage, the mixed powder may undergo additional processes, such as milling (sieving to adjust particle size) and granulation (improves compression and uniformity).
Direct Compression to Form the Tablet
Compression is the most critical process in tablet manufacturing, where an accurately measured amount of the mixed powder is filled into molds (dies) and subjected to high pressures. This process is automated using a tablet press, where two punches compress the powder within the die, creating a fused and compact tablet.
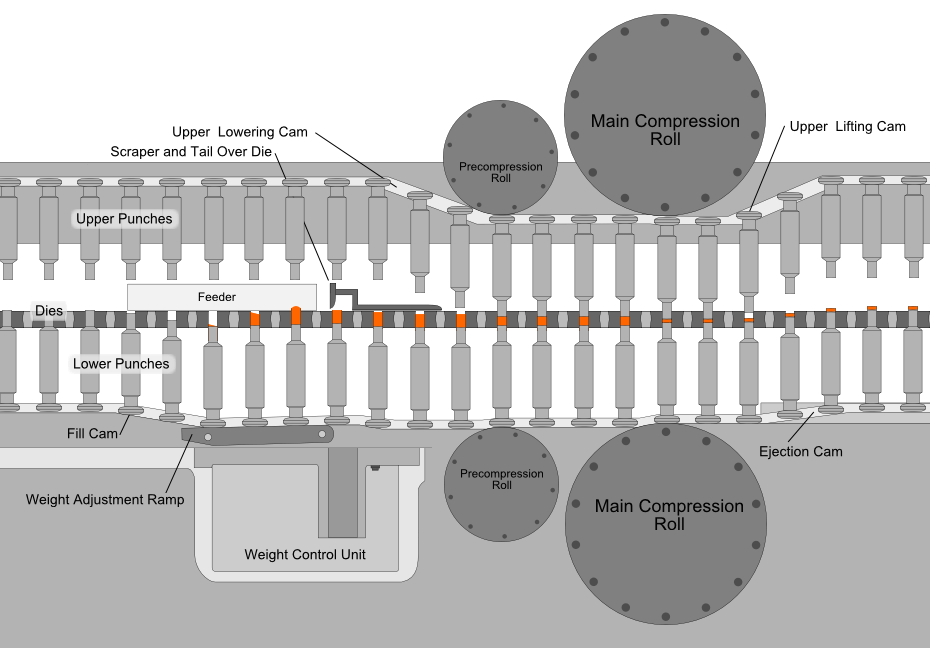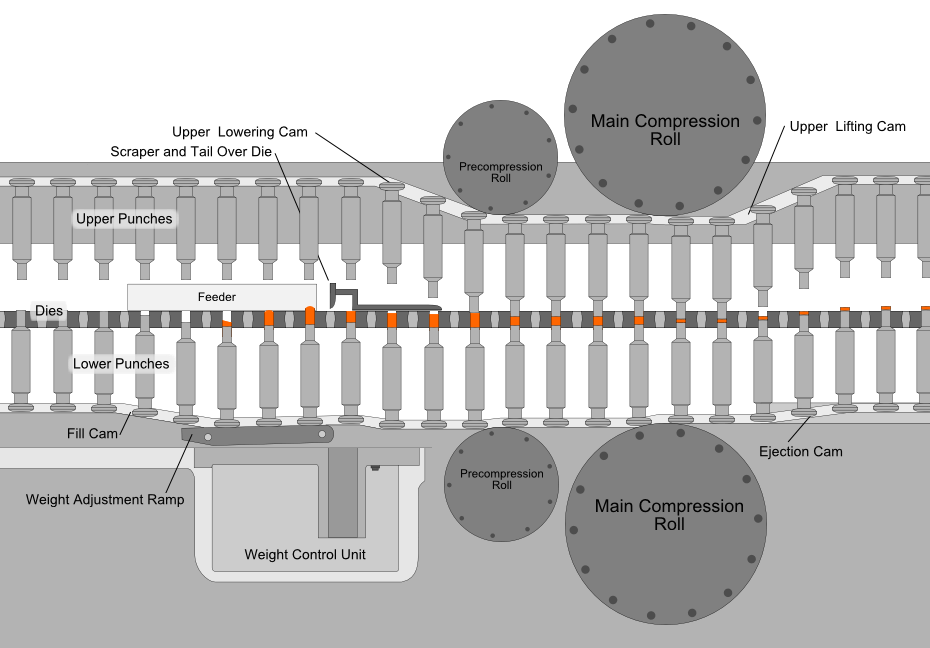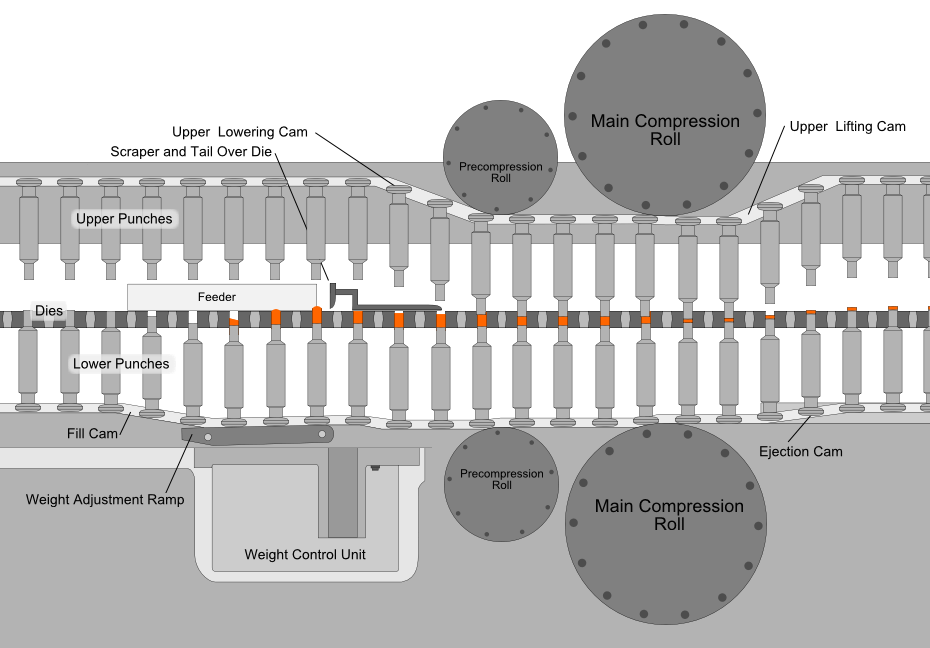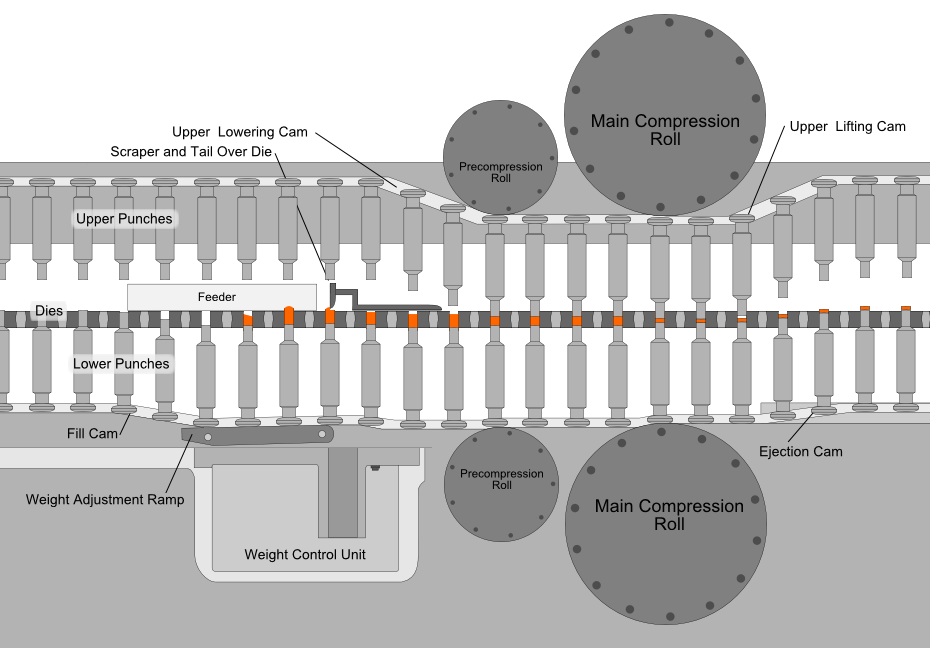
The force used to compress the powder is important; too much force causes the tablet to become dense and may not disintegrate properly in the patient’s stomach, too little force and the tablet may come apart during storage and transport.
The most common equipment for direct compression today is the rotary tablet press machine (like the one shown below). The compression roll revolves around a circular formation of molds while the mixture is fed from above, speeding up the tablet manufacturing process.

Coating the Finished Tablet
Coatings are applied to the tablet after it has taken shape, usually to mask the taste of bitter ingredients. These coatings are generally waxes or cellulose polymers, which are colorless, tasteless and have minimal effect on the overall properties of the tablet. Flavorings or artificial sweeteners are sometimes used in the coating if the taste of the tablet is exceptionally unpleasant.
Packaging for Storage and Transport
Finally, the completed tablet is ready to be sorted and packed. Some advantages of manufacturing tablet dosage forms is that they’re easy to package and have much longer shelf lives compared with liquid dosage forms. Blister packs made of aluminium and plastic are the cheapest option to protect the tablet, since patients only puncture the airtight blister right before taking it.
Plastic and glass bottles offer better protection during storage and transport but are more expensive to produce. The blister packs or bottles are then packed into bigger boxes, ready for shipping. It is a regulatory requirement that all medicines come with a label on the box, stating the amount of active ingredient present in the tablet and its possible side effects.
Important Factors in Tablet Design
Ingredients Affect Drug Behavior
When designing tablets, research teams must carefully choose the ingredients because the mixture affects the rate and location of the drug’s release in the patient’s body. Studying how the body breaks down the drug is a field called pharmacokinetics.
For example, we want to deliver a 100 mg drug dose to a patient. We produce two tablets manufactured in the same manner, one mixed with 900 mg of lactose (a sugar found in milk) and the other mixed with 900 mg of microcrystalline cellulose (a processed form of cellulose, a key molecule found in plants).
When the patient takes the drug, the tablet with the lactose filler will disintegrate first, as lactose is more soluble in the stomach’s gastric juices. Microcrystalline cellulose is insoluble in this acidic environment, holding the tablet in shape until it reaches the higher pH of the intestines. Researchers use dissolution machines like the one shown below to study how long different tablet formulations take to disintegrate in the body.

Manufacturing Costs and Sustainability
Cost is a critical factor in tablet manufacturing, as it affects the pharmaceutical company’s bottom line and—more importantly—the price patients will pay for the drug. Tablet compression is the most efficient way to produce drugs in terms of processing time per unit, labor costs and equipment costs. The excipients used in tablets are also, on average, 3 times cheaper than those used in making capsules.
Producing tablets also creates little waste, since direct compression makes use of all the ingredients to form the final product. No solvents are used in this process, either, improving safety and sustainability in line with the principles of Green Chemistry.
Tablet manufacturing technology has been around since the 1930s, culminating in ultra-efficient high-speed techniques available today that can produce up to 1 million tablets every hour! With the cost of developing a drug averaging US$2 billion, it makes financial sense for companies to prefer tablets over other dosage forms.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.




