Pharmaceutical AbbVie’s Humira for the treatment of rheumatoid arthritis recorded sales of USD 16 billion in 2016 alone. As the pharmaceutical industry as a whole looks beyond small molecules to biologicals as therapeutics, there has been increasing interest and investment in research in the biotech sector. In this post, a new approach to synthesizing small peptides is outlined, featuring an abstract written by a chemist doing active research in this area.
The abstract for this article has been submitted by Kay Yue (wenxiaoy@student.unimelb.edu.au), a 2nd year Masters student at the University of Melbourne. Contact us if you want to learn more about her research!
Biologic Drugs
Replacing the Small Molecule Drug
The pharmaceutical industry is undergoing a radical shift in its approach to drugs. Traditional small molecule therapeutics that have dominated the pharmaceutical industry are slowly being ousted by biologic drugs. Biologics encompasses therapies such as gene therapy, vaccines, monoclonal antibodies, and any drug that has its origins of a biological nature.

Biologic drugs have shown tremendous potential in the drug development landscape, with higher preclinical and clinical trial success rates than small molecule therapeutics. This is because they generally have higher specificities and efficacy while exhibiting lower toxicities and adverse effects.
Challenges Facing Biologic Drugs
It isn’t all rainbows and butterflies in the world of biologics, however. One of the main challenges facing the pharmaceutical industry is the inconsistencies that come with manufacturing products of biologic origin.
Traditional small molecules adhere to well-define chemical reactions. That’s why manufacturing conditions can vary, as long as the product can be analyzed and deemed acceptable by guidelines.
Compared to small molecules, however, biologic drugs (‘large’ molecules) require a completely different approach to large scale manufacturing. Due to their origin from living cells or tissue, processes are much harder to control. Extensive testing is required to ensure that bacterial toxins are not present in the final product.
Furthermore, purification and analysis can be difficult to the point where some say that “the product is the process”. Because of these difficulties, the consistency of a biological therapeutic is maintained by ensuring working manufacturing processes are relatively unchanged over time. Now, imagine setting up a new plant to manufacture these drugs! A real nightmare!
But what if peptides can be synthesized from start to finish, and analyzed along the way? Short-chain peptides fall into this category, in which single peptides can be bonded one at a time to give the required product, combining the efficacy of a biological and the synthetic precision of a small molecule drug.
Breakthrough in Biotechnology
Peptides as Therapeutics
Peptides have shown potent antiviral and antitumor activities; the last decade has seen an increase in the number of approvals for peptide-based drugs. Properties such as selectivity, stability and toxicity are important considerations in drug design.
Small cyclic peptides—compared to linear ones—tend to have higher therapeutic potential. This is because they have a more well-defined structure, stronger binding affinity and better resistance to digestion by enzymes in the body.
However, the chemical synthesis of small cyclic peptides is challenging as energetically unfavorable constrained ring structures are required. There is still no known general method to produce small cyclic peptides with plausible yields.

Research into Cyclic Peptides
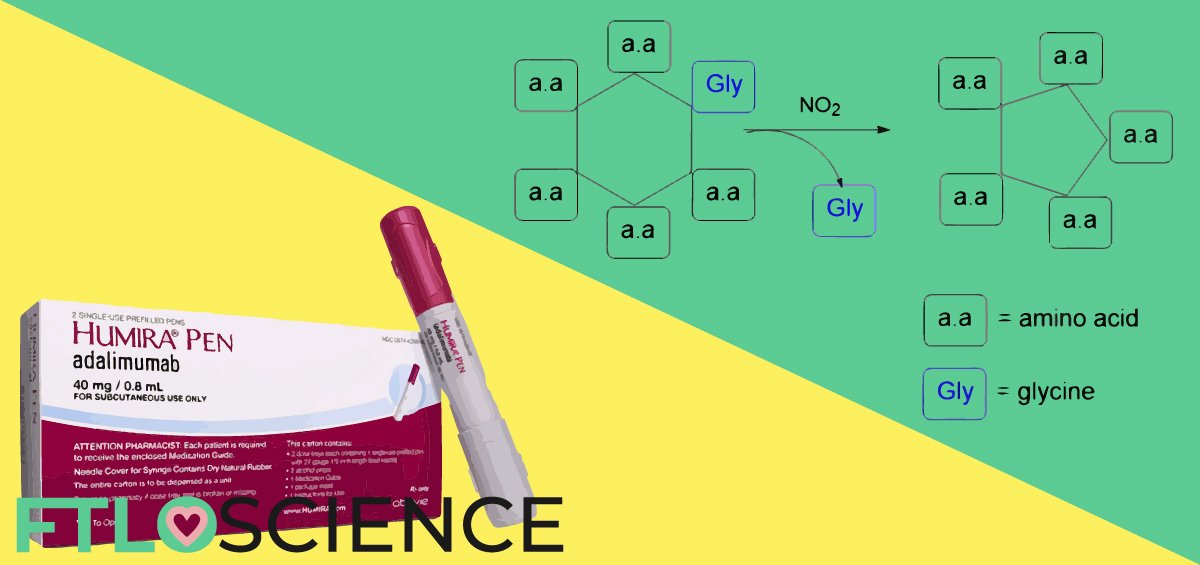
We are interested in developing new strategies for synthesizing small cyclic peptides. Our proposed method is by ring contraction of larger cyclic peptides by the removal of specific amino acid residues. Our preliminary work suggests that nitrogen dioxide has the ability to shorten linear peptide chains by one amino acid (Figure 1).
To probe the selectivity of the excision in linear systems, we have tested several linear peptides of various lengths. Our results show that the excision of an amino acid residue is governed by steric bulk, as the least spatially hindered residue, i.e., glycine, is preferentially kicked out.
Current research involves the optimization of the reaction’s outcome by altering various conditions such as temperature, reaction time and reagent concentrations.

Our next objective will be the application of this excision method to the synthesis of cyclic peptides. We expect this new approach to induce a ring contraction that allows the generation of small cyclic peptides from larger ones (Figure 2).
The reaction conditions will also be optimized in order to maximize the yield. This novel strategy will enable us to easily access therapeutically useful peptide-based drugs, advancing the Australian biotechnology industry.
About the Author

Sean is a consultant for clients in the pharmaceutical industry and is an associate lecturer at La Trobe University, where unfortunate undergrads are subject to his ramblings on chemistry and pharmacology.